Multifaceted N-Degron Recognition and Ubiquitylation by GID/CTLH E3 Ligases.
Chrustowicz, J., Sherpa, D., Teyra, J., Loke, M.S., Popowicz, G.M., Basquin, J., Sattler, M., Prabu, J.R., Sidhu, S.S., Schulman, B.A.(2022) J Mol Biology 434: 167347-167347
- PubMed: 34767800
- DOI: https://doi.org/10.1016/j.jmb.2021.167347
- Primary Citation of Related Structures:
7Q4Y, 7Q50, 7Q51 - PubMed Abstract:
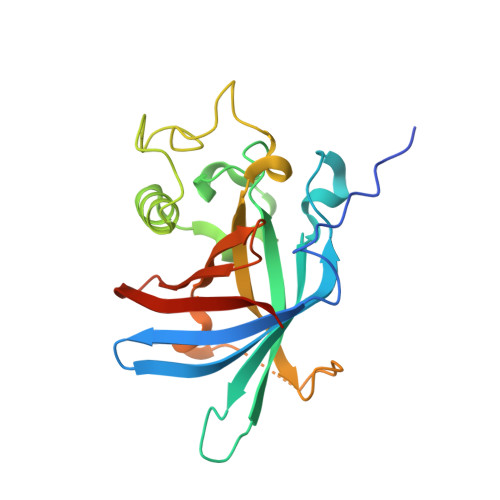
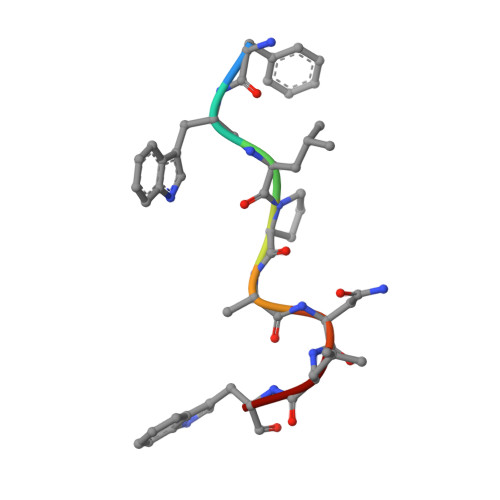
N-degron E3 ubiquitin ligases recognize specific residues at the N-termini of substrates. Although molecular details of N-degron recognition are known for several E3 ligases, the range of N-terminal motifs that can bind a given E3 substrate binding domain remains unclear. Here, we discovered capacity of Gid4 and Gid10 substrate receptor subunits of yeast "GID"/human "CTLH" multiprotein E3 ligases to tightly bind a wide range of N-terminal residues whose recognition is determined in part by the downstream sequence context. Screening of phage displaying peptide libraries with exposed N-termini identified novel consensus motifs with non-Pro N-terminal residues binding Gid4 or Gid10 with high affinity. Structural data reveal that conformations of flexible loops in Gid4 and Gid10 complement sequences and folds of interacting peptides. Together with analysis of endogenous substrate degrons, the data show that degron identity, substrate domains harboring targeted lysines, and varying E3 ligase higher-order assemblies combinatorially determine efficiency of ubiquitylation and degradation.
- Department of Molecular Machines and Signaling, Max Planck Institute of Biochemistry, 82152 Martinsried, Germany. Electronic address: https://twitter.com/chrustowicz_j.
Organizational Affiliation: