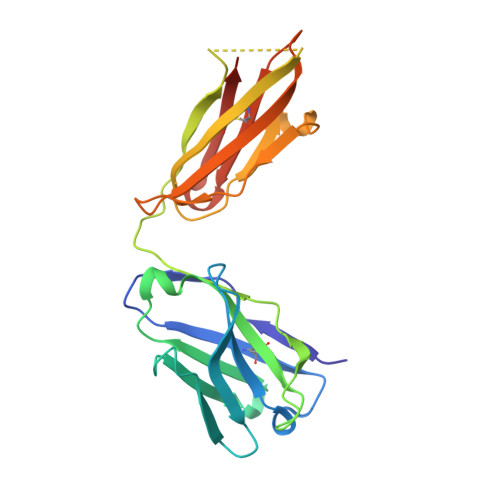
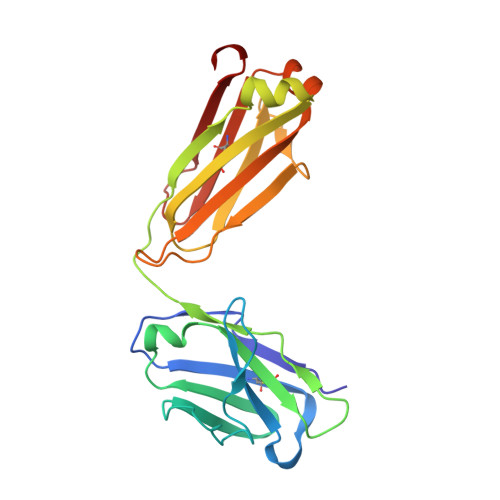
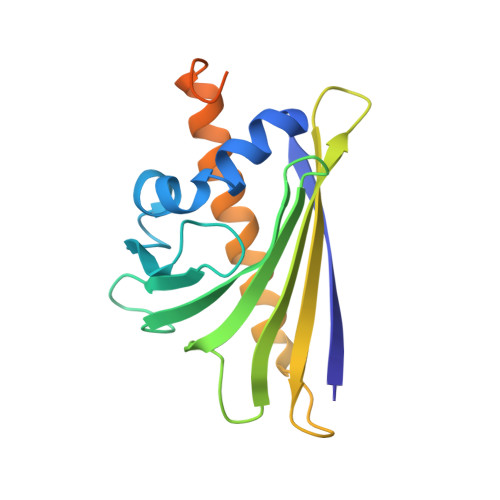
Targeting immunodominant Bet v 1 epitopes with monoclonal antibodies prevents the birch allergic response.
Atanasio, A., Franklin, M.C., Kamat, V., Hernandez, A.R., Badithe, A., Ben, L.H., Jones, J., Bautista, J., Yancopoulos, G.D., Olson, W., Murphy, A.J., Sleeman, M.A., Orengo, J.M.(2022) J Allergy Clin Immunol 149: 200-211
- PubMed: 34126155
- DOI: https://doi.org/10.1016/j.jaci.2021.05.038
- Primary Citation of Related Structures:
7MXL, 7N0U, 7N0V - PubMed Abstract:
Blocking the major cat allergen, Fel d 1, with mAbs was effective in preventing an acute cat allergic response. This study sought to extend the allergen-specific antibody approach and demonstrate that a combination of mAbs targeting Bet v 1, the immunodominant and most abundant allergenic protein in birch pollen, can prevent the birch allergic response. Bet v 1-specific mAbs, REGN5713, REGN5714, and REGN5715, were isolated using the VelocImmune platform. Surface plasmon resonance, x-ray crystallography, and cryo-electron microscopy determined binding kinetics and structural data. Inhibition of IgE-binding, basophil activation, and mast cell degranulation were assessed via blocking ELISA, flow cytometry, and the passive cutaneous anaphylaxis mouse model. REGN5713, REGN5714, and REGN5715 bind with high affinity and noncompetitively to Bet v 1. A cocktail of all 3 antibodies, REGN5713/14/15, blocks IgE binding to Bet v 1 and inhibits Bet v 1- and birch pollen extract-induced basophil activation ex vivo and mast cell degranulation in vivo. Crystal structures of the complex of Bet v 1 with immunoglobulin antigen-binding fragments of REGN5713 or REGN5715 show distinct interaction sites on Bet v 1. Cryo-electron microscopy reveals a planar and roughly symmetrical complex formed by REGN5713/14/15 bound to Bet v 1. These data confirm the immunodominance of Bet v 1 in birch allergy and demonstrate blockade of the birch allergic response with REGN5713/14/15. Structural analyses show simultaneous binding of REGN5713, REGN5714, and REGN5715 with substantial areas of Bet v 1 exposed, suggesting that targeting specific epitopes is sufficient to block the allergic response.
- Regeneron Pharmaceuticals, Inc, Tarrytown, NY.
Organizational Affiliation: