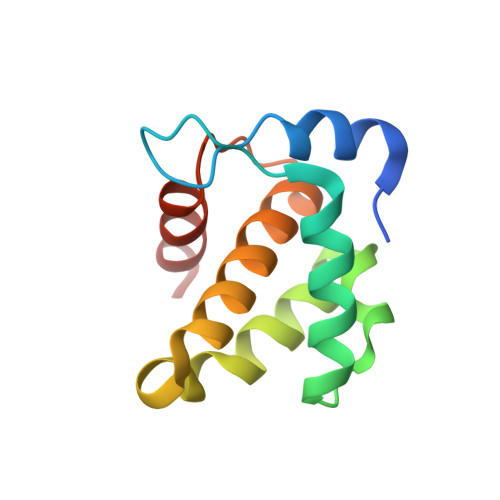
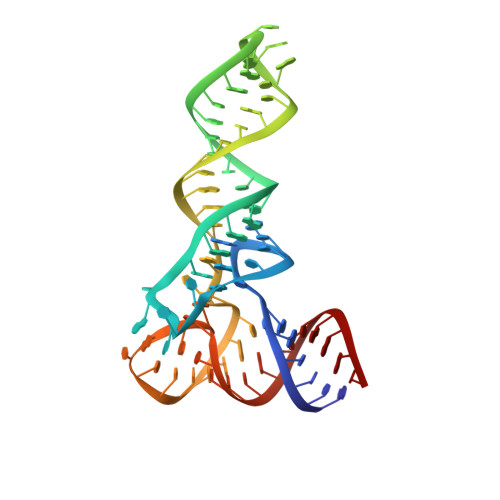
HIV-1 matrix-tRNA complex structure reveals basis for host control of Gag localization.
Bou-Nader, C., Muecksch, F., Brown, J.B., Gordon, J.M., York, A., Peng, C., Ghirlando, R., Summers, M.F., Bieniasz, P.D., Zhang, J.(2021) Cell Host Microbe 29: 1421
- PubMed: 34384537
- DOI: https://doi.org/10.1016/j.chom.2021.07.006
- Primary Citation of Related Structures:
7MRL - PubMed Abstract:
The HIV-1 virion structural polyprotein, Gag, is directed to particle assembly sites at the plasma membrane by its N-terminal matrix (MA) domain. MA also binds to host tRNAs. To understand the molecular basis of MA-tRNA interaction and its potential function, we present a co-crystal structure of HIV-1 MA-tRNA Lys3 complex. The structure reveals a specialized group of MA basic and aromatic residues preconfigured to recognize the distinctive structure of the tRNA elbow. Mutational, cross-linking, fluorescence, and NMR analyses show that the crystallographically defined interface drives MA-tRNA binding in solution and living cells. The structure indicates that MA is unlikely to bind tRNA and membrane simultaneously. Accordingly, single-amino-acid substitutions that abolish MA-tRNA binding caused striking redistribution of Gag to the plasma membrane and reduced HIV-1 replication. Thus, HIV-1 exploits host tRNAs to occlude a membrane localization signal and control the subcellular distribution of its major structural protein.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD 20892, USA.
Organizational Affiliation: