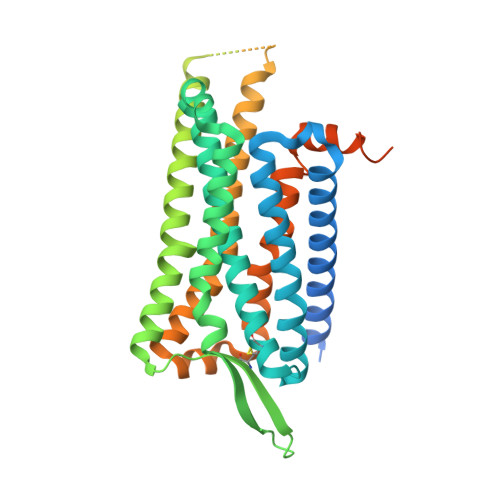
Structures of the human cholecystokinin 1 (CCK1) receptor bound to Gs and Gq mimetic proteins provide insight into mechanisms of G protein selectivity.
Mobbs, J.I., Belousoff, M.J., Harikumar, K.G., Piper, S.J., Xu, X., Furness, S.G.B., Venugopal, H., Christopoulos, A., Danev, R., Wootten, D., Thal, D.M., Miller, L.J., Sexton, P.M.(2021) PLoS Biol 19: e3001295-e3001295
- PubMed: 34086670
- DOI: https://doi.org/10.1371/journal.pbio.3001295
- Primary Citation of Related Structures:
7MBX, 7MBY - PubMed Abstract:
G protein-coupled receptors (GPCRs) are critical regulators of cellular function acting via heterotrimeric G proteins as their primary transducers with individual GPCRs capable of pleiotropic coupling to multiple G proteins. Structural features governing G protein selectivity and promiscuity are currently unclear. Here, we used cryo-electron microscopy (cryo-EM) to determine structures of the cholecystokinin (CCK) type 1 receptor (CCK1R) bound to the CCK peptide agonist, CCK-8 and 2 distinct transducer proteins, its primary transducer Gq, and the more weakly coupled Gs. As seen with other Gq/11-GPCR complexes, the Gq-α5 helix (αH5) bound to a relatively narrow pocket in the CCK1R core. Surprisingly, the backbone of the CCK1R and volume of the G protein binding pocket were essentially equivalent when Gs was bound, with the Gs αH5 displaying a conformation that arises from "unwinding" of the far carboxyl-terminal residues, compared to canonically Gs coupled receptors. Thus, integrated changes in the conformations of both the receptor and G protein are likely to play critical roles in the promiscuous coupling of individual GPCRs.
- Drug Discovery Biology Theme, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville, Victoria, Australia.
Organizational Affiliation: