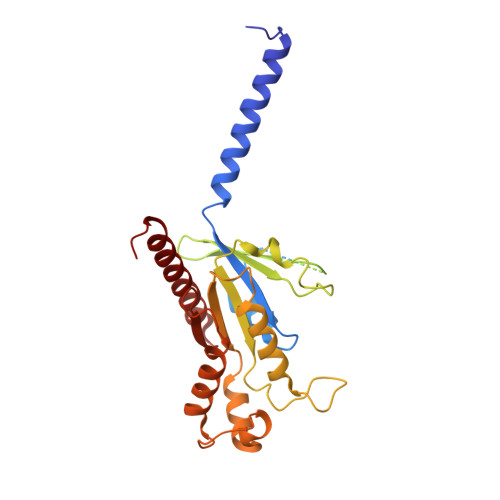
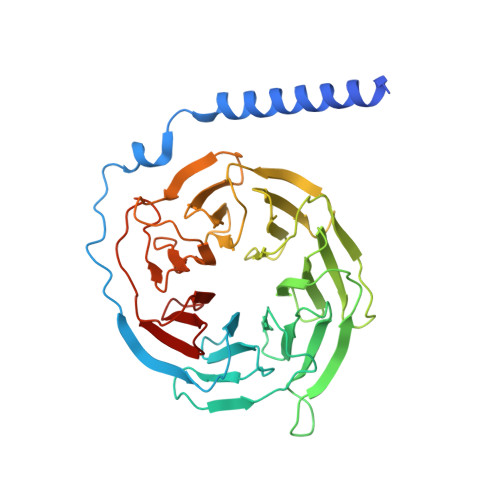
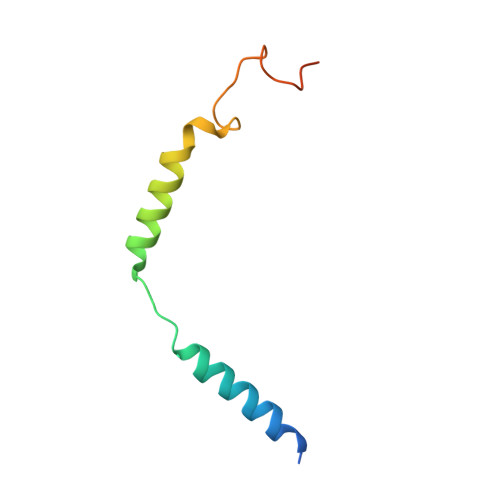
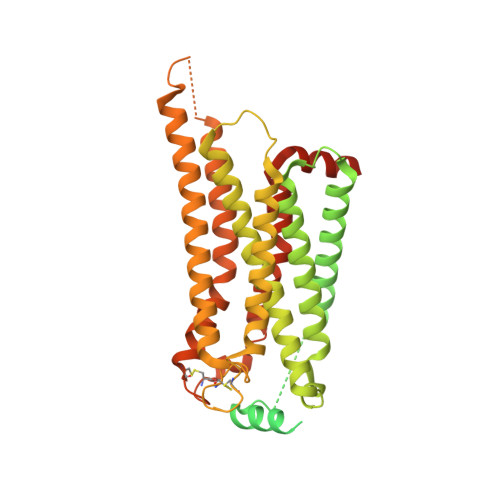
Structures of signaling complexes of lipid receptors S1PR1 and S1PR5 reveal mechanisms of activation and drug recognition.
Yuan, Y., Jia, G., Wu, C., Wang, W., Cheng, L., Li, Q., Li, Z., Luo, K., Yang, S., Yan, W., Su, Z., Shao, Z.(2021) Cell Res 31: 1263-1274
- PubMed: 34526663
- DOI: https://doi.org/10.1038/s41422-021-00566-x
- Primary Citation of Related Structures:
7EVY, 7EVZ, 7EW0, 7EW1, 7EW7 - PubMed Abstract:
Sphingosine-1-phosphate (S1P) is an important bioactive lipid molecule in cell membrane metabolism and binds to G protein-coupled S1P receptors (S1PRs) to regulate embryonic development, physiological homeostasis, and pathogenic processes in various organs. S1PRs are lipid-sensing receptors and are therapeutic targets for drug development, including potential treatment of COVID-19. Herein, we present five cryo-electron microscopy structures of S1PRs bound to diverse drug agonists and the heterotrimeric Gi protein. Our structural and functional assays demonstrate the different binding modes of chemically distinct agonists of S1PRs, reveal the mechanical switch that activates these receptors, and provide a framework for understanding ligand selectivity and G protein coupling.
- State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
Organizational Affiliation: