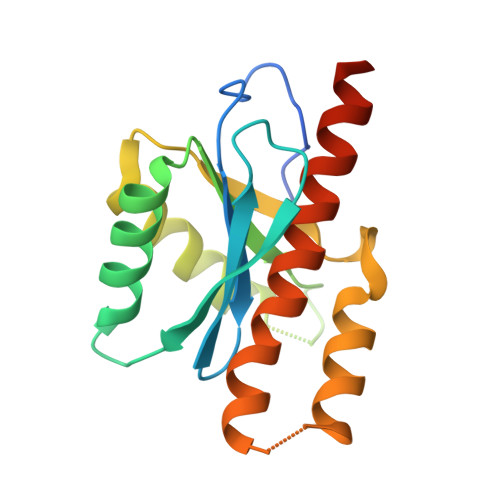
Novel insights into ATP-Stimulated Cleavage of branched DNA and RNA Substrates through Structure-Guided Studies of the Holliday Junction Resolvase RuvX.
Thakur, M., Mohan, D., Singh, A.K., Agarwal, A., Gopal, B., Muniyappa, K.(2021) J Mol Biology 433: 167014-167014
- PubMed: 33933468
- DOI: https://doi.org/10.1016/j.jmb.2021.167014
- Primary Citation of Related Structures:
7ESS - PubMed Abstract:
Much of our understanding of the homologous recombination (HR) machinery hinges on studies using Escherichia coli as a model organism. Interestingly enough, studies on the HR machinery in different bacterial species casts doubt on the universality of the E. coli paradigm. The human pathogen Mycobacterium tuberculosis encodes two Holliday junction (HJ)-resolvase paralogues, namely RuvC and RuvX; however, insights into their structural features and functional relevance is still limited. Here, we report on structure-guided functional studies of the M. tuberculosis RuvX HJ resolvase (MtRuvX). The crystalline MtRuvX is a dimer in the asymmetric unit, and each monomer has a RNAse H fold vis-à-vis RuvC-like nucleases. Interestingly, MtRuvX also contains some unique features, including the residues essential for ATP binding/coordination of Mg 2+ ions. Indeed, MtRuvX exhibited an intrinsic, robust ATPase activity, which was further accentuated by DNA cofactors. Structure-guided substitutions of single residues at the ATP binding/Mg 2+ coordination sites while markedly attenuating the ATPase activity completely abrogated HJ cleavage, indicating an unanticipated relationship between ATP hydrolysis and DNA cleavage. However, the affinity of ATPase-deficient mutants for the HJ was not impaired. Contrary to RuvC, MtRuvX exhibits relaxed substrate specificity, cleaving a variety of branched DNA/RNA substrates. Notably, ATP hydrolysis plays a regulatory role, rendering MtRuvX from a canonical HJ resolvase to a DNA/RNA non-sequence specific endonuclease, indicating a link between HJ resolvase and nucleic acid metabolism. These findings provide novel insights into the structure and dual-functional activities of MtRuvX, and suggest that it may play an important role in DNA/RNA metabolism.
- Department of Biochemistry, Indian Institute of Science, Bangalore 560 012, India.
Organizational Affiliation: