Characterization of a Conformation-Restricted Amyloid beta Peptide and Immunoreactivity of Its Antibody in Human AD brain.
Kageyama, Y., Irie, Y., Matsushima, Y., Segawa, T., Bellier, J.P., Hidaka, K., Sugiyama, H., Kaneda, D., Hashizume, Y., Akatsu, H., Miki, K., Kita, A., Walker, D.G., Irie, K., Tooyama, I.(2021) ACS Chem Neurosci 12: 3418-3432
- PubMed: 34464082
- DOI: https://doi.org/10.1021/acschemneuro.1c00416
- Primary Citation of Related Structures:
7E6P - PubMed Abstract:
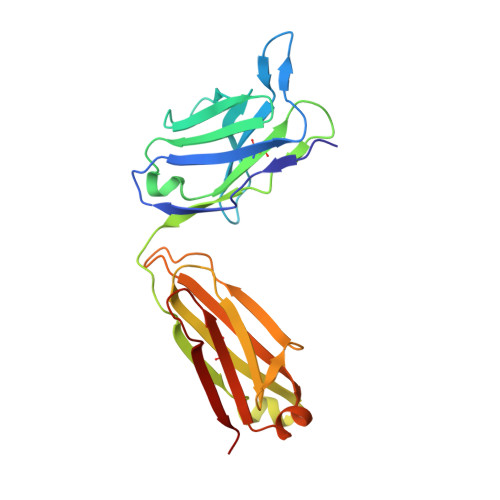
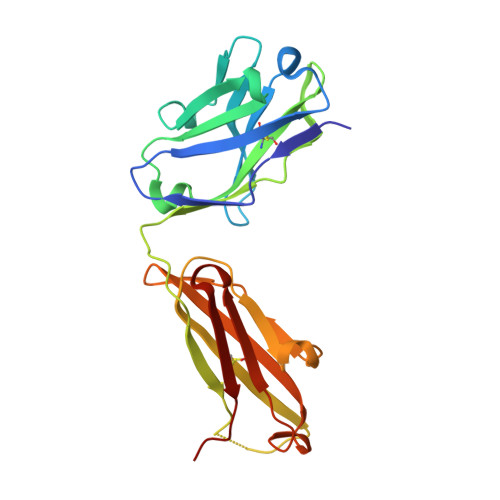
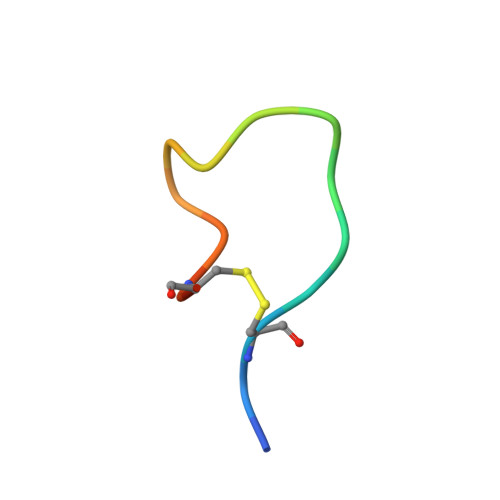
Characterization of amyloid β (Aβ) oligomers, the transition species present prior to the formation of Aβ fibrils and that have cytotoxicity, has become one of the major topics in the investigations of Alzheimer's disease (AD) pathogenesis. However, studying pathophysiological properties of Aβ oligomers is challenging due to the instability of these protein complexes in vitro . Here, we report that conformation-restricted Aβ42 with an intramolecular disulfide bond at positions 17 and 28 (SS-Aβ42) formed stable Aβ oligomers in vitro . Thioflavin T binding assays, nondenaturing gel electrophoresis, and morphological analyses revealed that SS-Aβ42 maintained oligomeric structure, whereas wild-type Aβ42 and the highly aggregative Aβ42 mutant with E22P substitution (E22P-Aβ42) formed Aβ fibrils. In agreement with these observations, SS-Aβ42 was more cytotoxic compared to the wild-type and E22P-Aβ42 in cell cultures. Furthermore, we developed a monoclonal antibody, designated TxCo-1, using the toxic conformation of SS-Aβ42 as immunogen. X-ray crystallography of the TxCo-1/SS-Aβ42 complex, enzyme immunoassay, and immunohistochemical studies confirmed the recognition site and specificity of TxCo-1 to SS-Aβ42. Immunohistochemistry with TxCo-1 antibody identified structures resembling senile plaques and vascular Aβ in brain samples of AD subjects. However, TxCo-1 immunoreactivity did not colocalize extensively with Aβ plaques identified with conventional Aβ antibodies. Together, these findings indicate that Aβ with a turn at positions 22 and 23, which is prone to form Aβ oligomers, could show strong cytotoxicity and accumulated in brains of AD subjects. The SS-Aβ42 and TxCo-1 antibody should facilitate understanding of the pathological role of Aβ with toxic conformation in AD.
- Molecular Neuroscience Research Center, Shiga University of Medical Science, Shiga 520-2192, Japan.
Organizational Affiliation: