Structural Basis for Peptide Binding of Alpha-N Terminal Methyltransferase from Saccharomyces cerevisiae
Zhang, H.Y., Kuang, Z., Xue, L., Yue, J., Khan, M.H., Zhu, Z., Niu, L.(2021) Crystallogr Rep 66: 1316-1321
Experimental Data Snapshot
Starting Model: experimental
View more details
(2021) Crystallogr Rep 66: 1316-1321
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Alpha N-terminal protein methyltransferase 1 | 232 | Saccharomyces cerevisiae | Mutation(s): 0 Gene Names: TAE1, NTM1, YBR261C, YBR1729 EC: 2.1.1.244 | 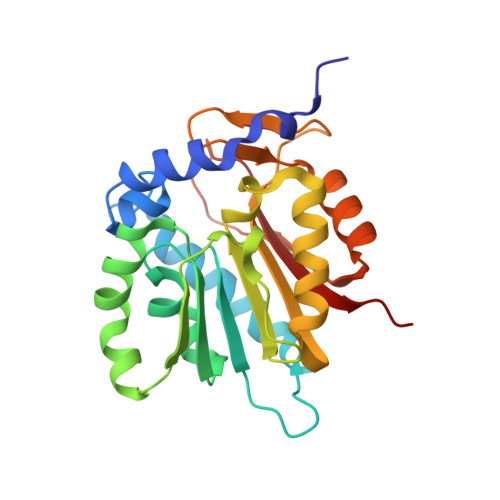 | |
UniProt | |||||
Find proteins for P38340 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P38340 Go to UniProtKB: P38340 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P38340 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Rps25A-peptide | B [auth D] | 6 | Saccharomyces cerevisiae | Mutation(s): 0 | 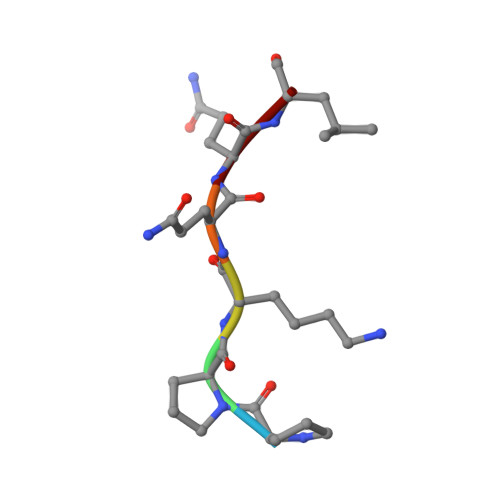 |
UniProt | |||||
Find proteins for Q3E792 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore Q3E792 Go to UniProtKB: Q3E792 | |||||
Entity Groups | |||||
| UniProt Group | Q3E792 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SAH (Subject of Investigation/LOI) Query on SAH | C [auth A] | S-ADENOSYL-L-HOMOCYSTEINE C14 H20 N6 O5 S ZJUKTBDSGOFHSH-WFMPWKQPSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 38.692 | α = 90 |
| b = 52.014 | β = 90 |
| c = 127.723 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | U1632124 to L.N. |
| Ministry of Science and Technology (MoST, China) | China | 2017YFA0503600 to L.N. |