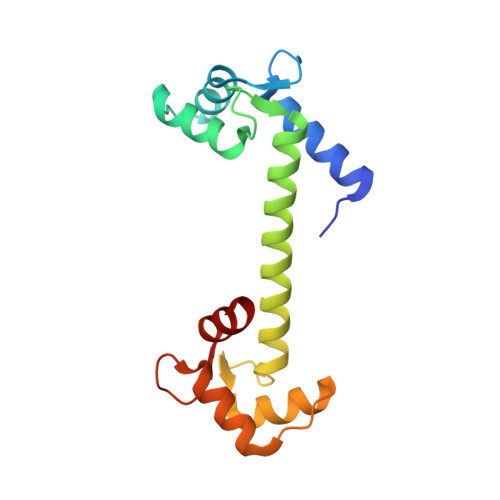
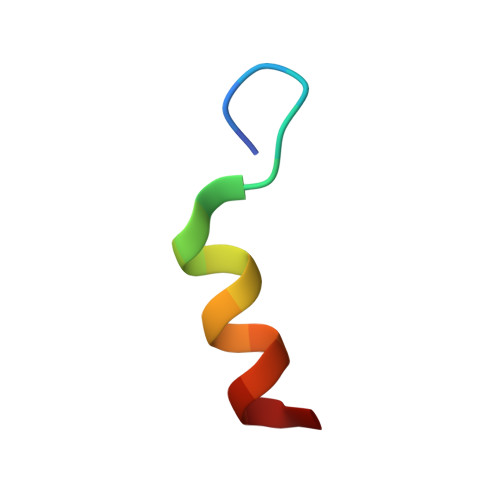
Calmodulin complexes with brain and muscle creatine kinase peptides.
Sprenger, J., Trifan, A., Patel, N., Vanderbeck, A., Bredfelt, J., Tajkhorshid, E., Rowlett, R., Lo Leggio, L., Akerfeldt, K.S., Linse, S.(2021) Curr Res Struct Biol 3: 121-132
- PubMed: 34235492
- DOI: https://doi.org/10.1016/j.crstbi.2021.05.001
- Primary Citation of Related Structures:
7BF1, 7BF2 - PubMed Abstract:
Calmodulin (CaM) is a ubiquitous Ca 2+ sensing protein that binds to and modulates numerous target proteins and enzymes during cellular signaling processes. A large number of CaM-target complexes have been identified and structurally characterized, revealing a wide diversity of CaM-binding modes. A newly identified target is creatine kinase (CK), a central enzyme in cellular energy homeostasis. This study reports two high-resolution X-ray structures, determined to 1.24 Å and 1.43 Å resolution, of calmodulin in complex with peptides from human brain and muscle CK, respectively. Both complexes adopt a rare extended binding mode with an observed stoichiometry of 1:2 CaM:peptide, confirmed by isothermal titration calorimetry, suggesting that each CaM domain independently binds one CK peptide in a Ca 2+ -depended manner. While the overall binding mode is similar between the structures with muscle or brain-type CK peptides, the most significant difference is the opposite binding orientation of the peptides in the N-terminal domain. This may extrapolate into distinct binding modes and regulation of the full-length CK isoforms. The structural insights gained in this study strengthen the link between cellular energy homeostasis and Ca 2+ -mediated cell signaling and may shed light on ways by which cells can 'fine tune' their energy levels to match the spatial and temporal demands.
- Department of Biochemistry and Structural Biology, Chemical Center, PO Box 124, SE-221 00, Lund, Sweden.
Organizational Affiliation: