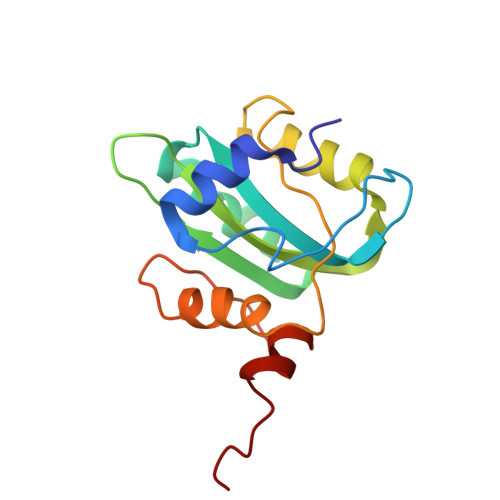
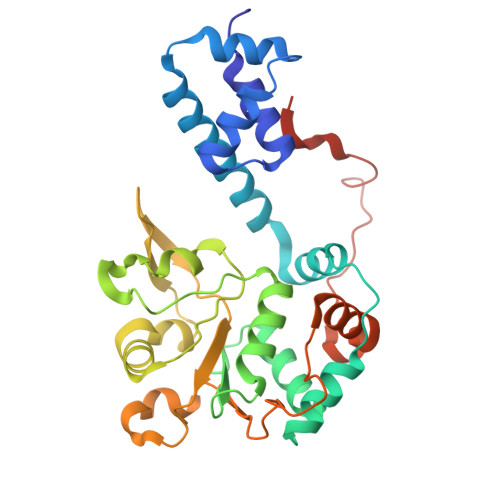
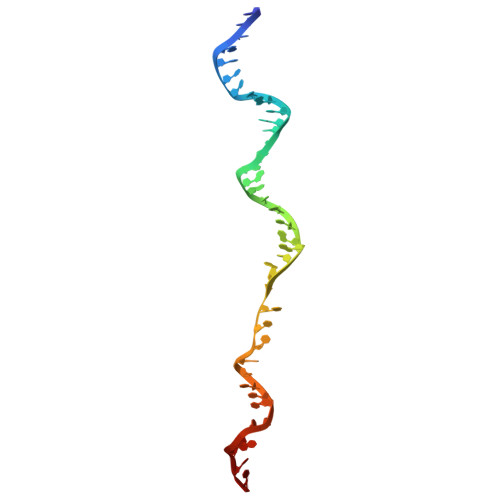
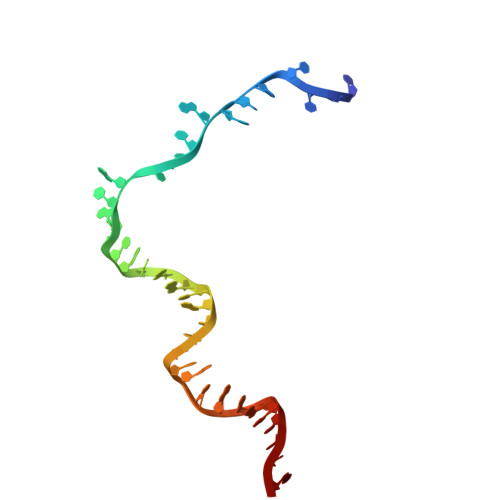
Unwinding of a DNA replication fork by a hexameric viral helicase.
Javed, A., Major, B., Stead, J.A., Sanders, C.M., Orlova, E.V.(2021) Nat Commun 12: 5535-5535
- PubMed: 34545080
- DOI: https://doi.org/10.1038/s41467-021-25843-6
- Primary Citation of Related Structures:
7APD - PubMed Abstract:
Hexameric helicases are motor proteins that unwind double-stranded DNA (dsDNA) during DNA replication but how they are optimised for strand separation is unclear. Here we present the cryo-EM structure of the full-length E1 helicase from papillomavirus, revealing all arms of a bound DNA replication fork and their interactions with the helicase. The replication fork junction is located at the entrance to the helicase collar ring, that sits above the AAA + motor assembly. dsDNA is escorted to and the 5´ single-stranded DNA (ssDNA) away from the unwinding point by the E1 dsDNA origin binding domains. The 3´ ssDNA interacts with six spirally-arranged β-hairpins and their cyclical top-to-bottom movement pulls the ssDNA through the helicase. Pulling of the RF against the collar ring separates the base-pairs, while modelling of the conformational cycle suggest an accompanying movement of the collar ring has an auxiliary role, helping to make efficient use of ATP in duplex unwinding.
- Department of Biological Sciences, Birkbeck College, Institute of Structural and Molecular Biology, Malet Street, London, WC1E 7HX, UK.
Organizational Affiliation: