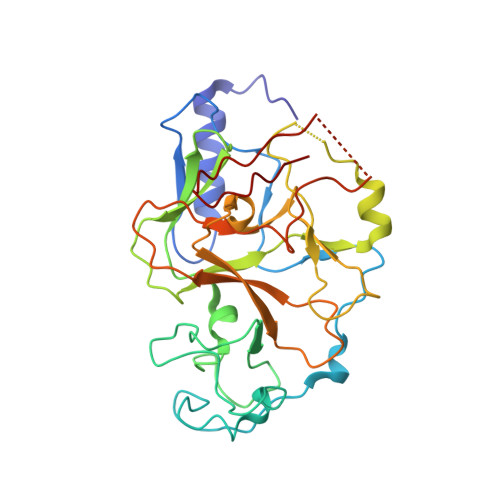
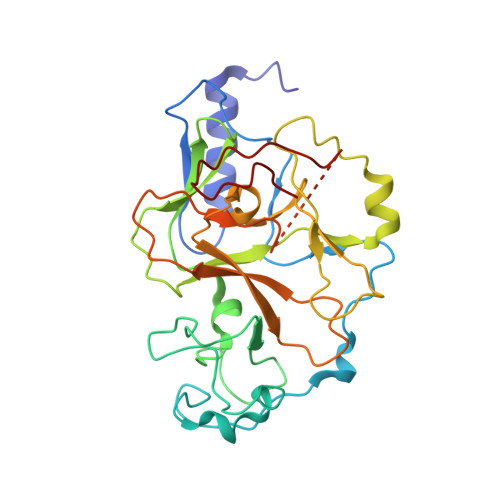
SUV39 SET domains mediate crosstalk of heterochromatic histone marks.
Stirpe, A., Guidotti, N., Northall, S.J., Kilic, S., Hainard, A., Vadas, O., Fierz, B., Schalch, T.(2021) Elife 10
- PubMed: 34524082
- DOI: https://doi.org/10.7554/eLife.62682
- Primary Citation of Related Structures:
6Z2A - PubMed Abstract:
The SUV39 class of methyltransferase enzymes deposits histone H3 lysine 9 di- and trimethylation (H3K9me2/3), the hallmark of constitutive heterochromatin. How these enzymes are regulated to mark specific genomic regions as heterochromatic is poorly understood. Clr4 is the sole H3K9me2/3 methyltransferase in the fission yeast Schizosaccharomyces pombe, and recent evidence suggests that ubiquitination of lysine 14 on histone H3 (H3K14ub) plays a key role in H3K9 methylation. However, the molecular mechanism of this regulation and its role in heterochromatin formation remain to be determined. Our structure-function approach shows that the H3K14ub substrate binds specifically and tightly to the catalytic domain of Clr4, and thereby stimulates the enzyme by over 250-fold. Mutations that disrupt this mechanism lead to a loss of H3K9me2/3 and abolish heterochromatin silencing similar to clr4 deletion. Comparison with mammalian SET domain proteins suggests that the Clr4 SET domain harbors a conserved sensor for H3K14ub, which mediates licensing of heterochromatin formation.
- Department of Molecular Biology, Faculty of Science, University of Geneva, Geneva, Switzerland.
Organizational Affiliation: