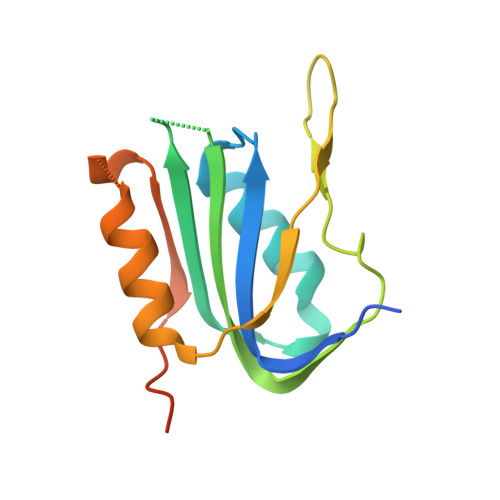
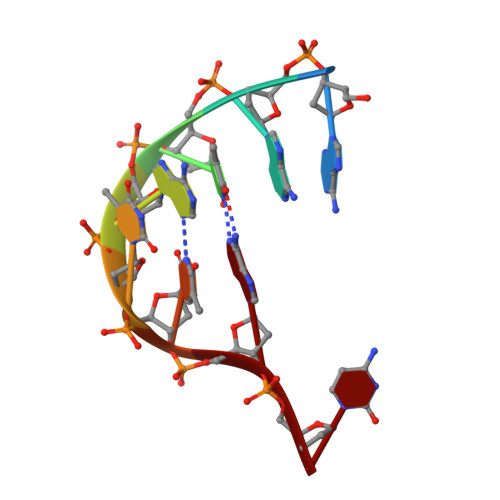
Molecular underpinnings of ssDNA specificity by Rep HUH-endonucleases and implications for HUH-tag multiplexing and engineering.
Tompkins, K.J., Houtti, M., Litzau, L.A., Aird, E.J., Everett, B.A., Nelson, A.T., Pornschloegl, L., Limon-Swanson, L.K., Evans, R.L., Evans, K., Shi, K., Aihara, H., Gordon, W.R.(2021) Nucleic Acids Res 49: 1046-1064
- PubMed: 33410911
- DOI: https://doi.org/10.1093/nar/gkaa1248
- Primary Citation of Related Structures:
6WDZ, 6WE0, 6WE1 - PubMed Abstract:
Replication initiator proteins (Reps) from the HUH-endonuclease superfamily process specific single-stranded DNA (ssDNA) sequences to initiate rolling circle/hairpin replication in viruses, such as crop ravaging geminiviruses and human disease causing parvoviruses. In biotechnology contexts, Reps are the basis for HUH-tag bioconjugation and a critical adeno-associated virus genome integration tool. We solved the first co-crystal structures of Reps complexed to ssDNA, revealing a key motif for conferring sequence specificity and for anchoring a bent DNA architecture. In combination, we developed a deep sequencing cleavage assay, termed HUH-seq, to interrogate subtleties in Rep specificity and demonstrate how differences can be exploited for multiplexed HUH-tagging. Together, our insights allowed engineering of only four amino acids in a Rep chimera to predictably alter sequence specificity. These results have important implications for modulating viral infections, developing Rep-based genomic integration tools, and enabling massively parallel HUH-tag barcoding and bioconjugation applications.
- Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota Twin Cities, Minneapolis, MN 55455, USA.
Organizational Affiliation: