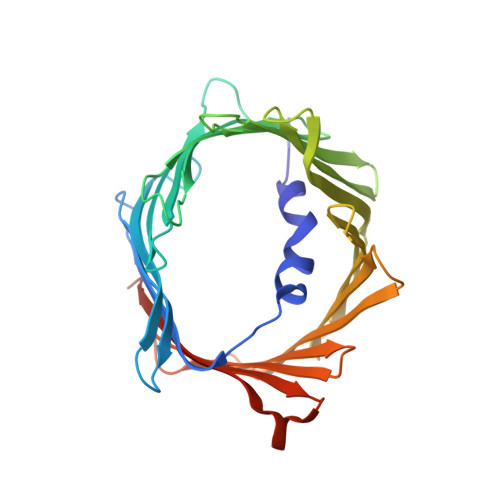
The Structural Basis for Low Conductance in the Membrane Protein VDAC upon beta-NADH Binding and Voltage Gating.
Bohm, R., Amodeo, G.F., Murlidaran, S., Chavali, S., Wagner, G., Winterhalter, M., Brannigan, G., Hiller, S.(2020) Structure 28: 206-214.e4
- PubMed: 31862297
- DOI: https://doi.org/10.1016/j.str.2019.11.015
- Primary Citation of Related Structures:
6TIQ, 6TIR - PubMed Abstract:
The voltage-dependent anion channel (VDAC) forms the primary diffusion pore of the outer mitochondrial membrane. In its apo form, VDAC adopts an open conformation with high conductance. States of lower conductance can be induced by ligand binding or the application of voltage. Here, we clarify at the atomic level how β-NADH binding leads to a low-conductance state and characterize the role of the VDAC N-terminal helix in voltage gating. A high-resolution NMR structure of human VDAC-1 with bound NADH, combined with molecular dynamics simulation show that β-NADH binding reduces the pore conductance sterically without triggering a structural change. Electrophysiology recordings of crosslinked protein variants and NMR relaxation experiments probing different time scales show that increased helix dynamics is present in the open state and that motions of the N-terminal helices are involved in the VDAC voltage gating mechanism.
- Biozentrum, University of Basel, Basel 4056, Switzerland.
Organizational Affiliation: