A trapped human PPM1A-phosphopeptide complex reveals structural features critical for regulation of PPM protein phosphatase activity.
Debnath, S., Kosek, D., Tagad, H.D., Durell, S.R., Appella, D.H., Acevedo, R., Grishaev, A., Dyda, F., Appella, E., Mazur, S.J.(2018) J Biological Chem 293: 7993-8008
- PubMed: 29602904
- DOI: https://doi.org/10.1074/jbc.RA117.001213
- Primary Citation of Related Structures:
6B67 - PubMed Abstract:
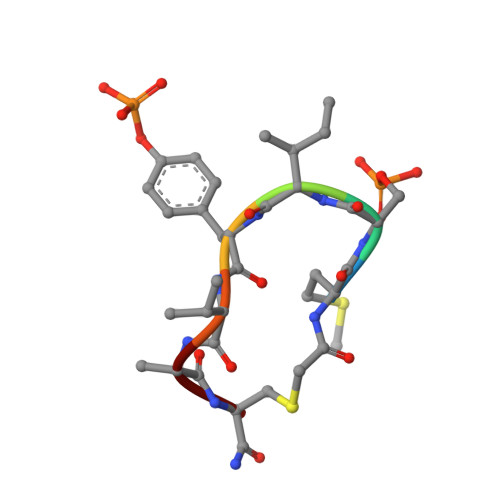
Metal-dependent protein phosphatases (PPM) are evolutionarily unrelated to other serine/threonine protein phosphatases and are characterized by their requirement for supplementation with millimolar concentrations of Mg 2+ or Mn 2+ ions for activity in vitro The crystal structure of human PPM1A (also known as PP2Cα), the first PPM structure determined, displays two tightly bound Mn 2+ ions in the active site and a small subdomain, termed the Flap, located adjacent to the active site. Some recent crystal structures of bacterial or plant PPM phosphatases have disclosed two tightly bound metal ions and an additional third metal ion in the active site. Here, the crystal structure of the catalytic domain of human PPM1A, PPM1A cat , complexed with a cyclic phosphopeptide, c(MpSIpYVA), a cyclized variant of the activation loop of p38 MAPK (a physiological substrate of PPM1A), revealed three metal ions in the active site. The PPM1A cat D146E-c(MpSIpYVA) complex confirmed the presence of the anticipated third metal ion in the active site of metazoan PPM phosphatases. Biophysical and computational methods suggested that complex formation results in a slightly more compact solution conformation through reduced conformational flexibility of the Flap subdomain. We also observed that the position of the substrate in the active site allows solvent access to the labile third metal-binding site. Enzyme kinetics of PPM1A cat toward a phosphopeptide substrate supported a random-order, bi-substrate mechanism, with substantial interaction between the bound substrate and the labile metal ion. This work illuminates the structural and thermodynamic basis of an innate mechanism regulating the activity of PPM phosphatases.
- Laboratory of Cell Biology, Center for Cancer Research, NCI, Bethesda, Maryland 20892.
Organizational Affiliation: