Structure-Function Studies of Two Yeast Homing Endonucleases that Evolved to Cleave Identical Targets with Dissimilar Rates and Specificities.
Nawimanage, R.R., Yuan, Z., Casares, M., Joshi, R., Lohman, J.R., Gimble, F.S.(2022) J Mol Biology 434: 167550-167550
- PubMed: 35317996
- DOI: https://doi.org/10.1016/j.jmb.2022.167550
- Primary Citation of Related Structures:
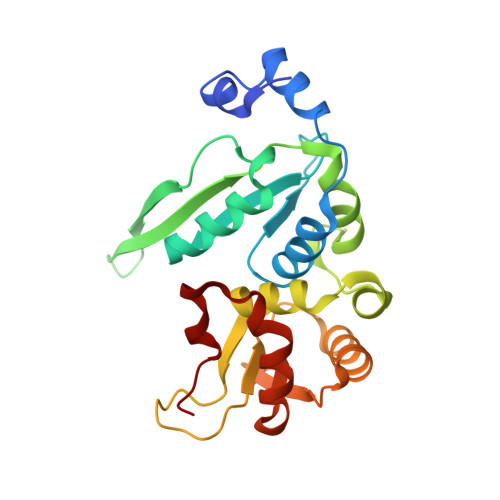
6X1J - PubMed Abstract:
The LAGLIDADG family of homing endonucleases (LHEs) bind to and cleave their DNA recognition sequences with high specificity. Much of our understanding for how these proteins evolve their specificities has come from studying LHE homologues. To gain insight into the molecular basis of LHE specificity, we characterized I-WcaI, the homologue of the Saccharomyces cerevisiae I-SceI LHE found in Wickerhamomyces canadensis. Although I-WcaI and I-SceI cleave the same recognition sequence, expression of I-WcaI, but not I-SceI, is toxic in bacteria. Toxicity suppressing mutations frequently occur at I-WcaI residues critical for activity and I-WcaI cleaves many more non-cognate sequences in the Escherichia coli genome than I-SceI, suggesting I-WcaI endonuclease activity is the basis of toxicity. In vitro, I-WcaI is a more active and a less specific endonuclease than I-SceI, again accounting for the observed toxicity in vivo. We determined the X-ray crystal structure of I-WcaI bound to its cognate target site and found that I-WcaI and I-SceI use residues at different positions to make similar base-specific contacts. Furthermore, in some regions of the DNA interface where I-WcaI specificity is lower, the protein makes fewer DNA contacts than I-SceI. Taken together, these findings demonstrate the plastic nature of LHE site recognition and suggest that I-WcaI and I-SceI are situated at different points in their evolutionary pathways towards acquiring target site specificity.
- Department of Biochemistry, Purdue University, West Lafayette, IN 47907, USA.
Organizational Affiliation: