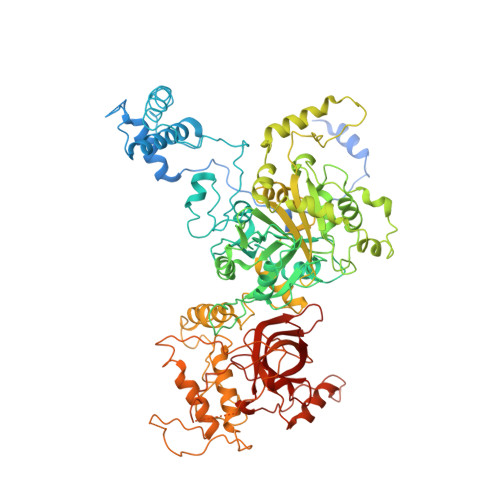
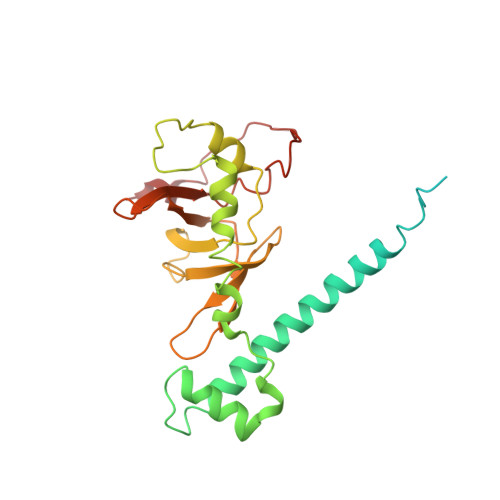
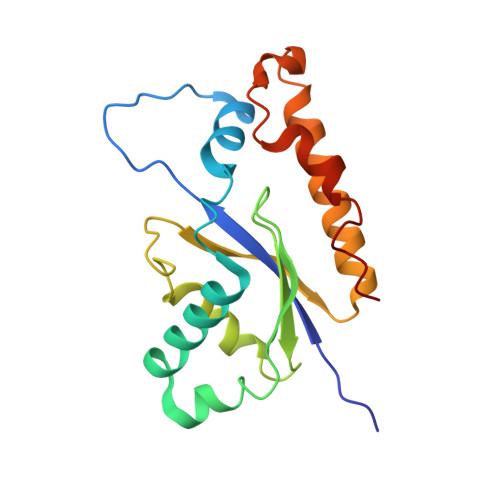
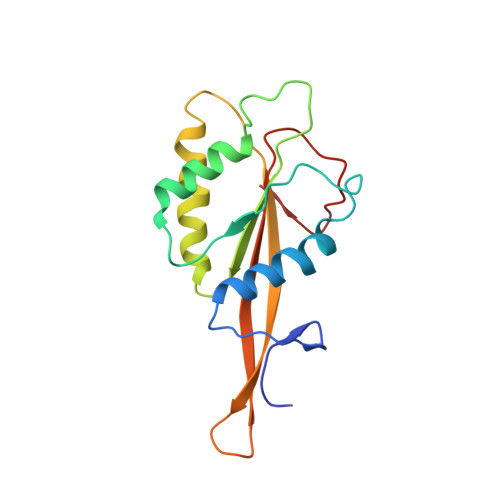
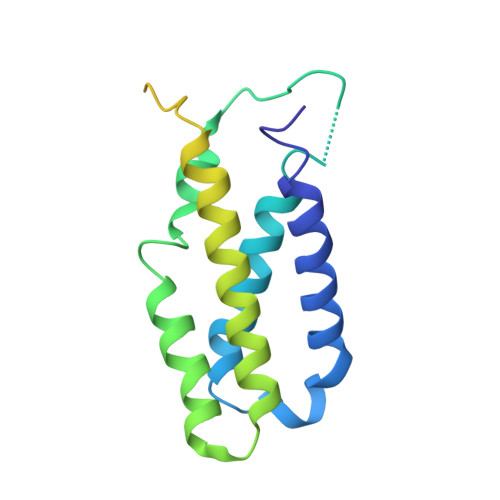
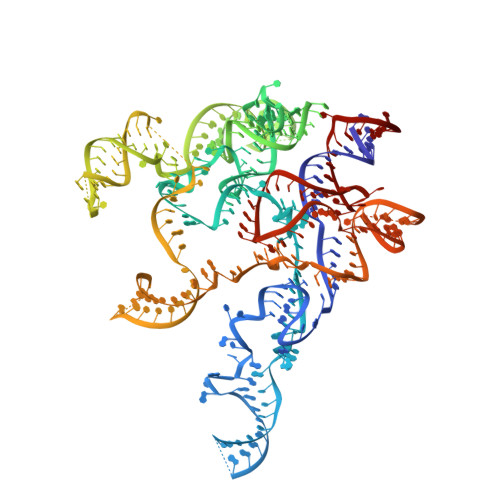
Cryo-EM structure of catalytic ribonucleoprotein complex RNase MRP.
Perederina, A., Li, D., Lee, H., Bator, C., Berezin, I., Hafenstein, S.L., Krasilnikov, A.S.(2020) Nat Commun 11: 3474-3474
- PubMed: 32651392
- DOI: https://doi.org/10.1038/s41467-020-17308-z
- Primary Citation of Related Structures:
6W6V - PubMed Abstract:
RNase MRP is an essential eukaryotic ribonucleoprotein complex involved in the maturation of rRNA and the regulation of the cell cycle. RNase MRP is related to the ribozyme-based RNase P, but it has evolved to have distinct cellular roles. We report a cryo-EM structure of the S. cerevisiae RNase MRP holoenzyme solved to 3.0 Å. We describe the structure of this 450 kDa complex, interactions between its components, and the organization of its catalytic RNA. We show that some of the RNase MRP proteins shared with RNase P undergo an unexpected RNA-driven remodeling that allows them to bind to divergent RNAs. Further, we reveal how this RNA-driven protein remodeling, acting together with the introduction of new auxiliary elements, results in the functional diversification of RNase MRP and its progenitor, RNase P, and demonstrate structural underpinnings of the acquisition of new functions by catalytic RNPs.
- Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, 16802, PA, USA.
Organizational Affiliation: