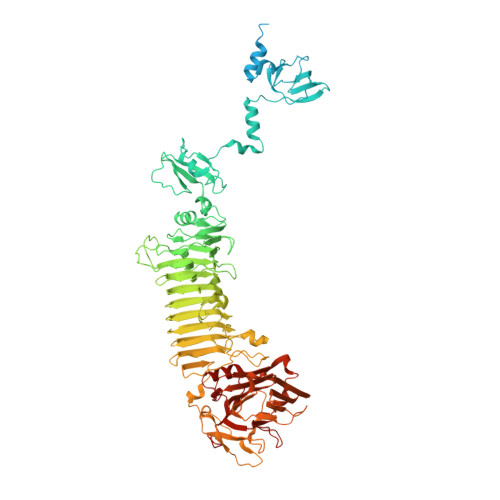
Structure and function of bacteriophage CBA120 ORF211 (TSP2), the determinant of phage specificity towards E. coli O157:H7.
Greenfield, J., Shang, X., Luo, H., Zhou, Y., Linden, S.B., Heselpoth, R.D., Leiman, P.G., Nelson, D.C., Herzberg, O.(2020) Sci Rep 10: 15402-15402
- PubMed: 32958885
- DOI: https://doi.org/10.1038/s41598-020-72373-0
- Primary Citation of Related Structures:
6W4Q - PubMed Abstract:
The genome of Escherichia coli O157:H7 bacteriophage vB_EcoM_CBA120 encodes four distinct tailspike proteins (TSPs). The four TSPs, TSP1-4, attach to the phage baseplate forming a branched structure. We report the 1.9 Å resolution crystal structure of TSP2 (ORF211), the TSP that confers phage specificity towards E. coli O157:H7. The structure shows that the N-terminal 168 residues involved in TSPs complex assembly are disordered in the absence of partner proteins. The ensuing head domain contains only the first of two fold modules seen in other phage vB_EcoM_CBA120 TSPs. The catalytic site resides in a cleft at the interface between adjacent trimer subunits, where Asp506, Glu568, and Asp571 are located in close proximity. Replacement of Asp506 and Asp571 for alanine residues abolishes enzyme activity, thus identifying the acid/base catalytic machinery. However, activity remains intact when Asp506 and Asp571 are mutated into asparagine residues. Analysis of additional site-directed mutants in the background of the D506N:D571N mutant suggests engagement of an alternative catalytic apparatus comprising Glu568 and Tyr623. Finally, we demonstrate the catalytic role of two interacting glutamate residues of TSP1, located in a cleft between two trimer subunits, Glu456 and Glu483, underscoring the diversity of the catalytic apparatus employed by phage vB_EcoM_CBA120 TSPs.
- Institute for Bioscience and Biotechnology Research, University of Maryland, 9600 Gudelsky Dr, Rockville, MD, 20850, USA.
Organizational Affiliation: