Identification of MLKL membrane translocation as a checkpoint in necroptotic cell death using Monobodies.
Petrie, E.J., Birkinshaw, R.W., Koide, A., Denbaum, E., Hildebrand, J.M., Garnish, S.E., Davies, K.A., Sandow, J.J., Samson, A.L., Gavin, X., Fitzgibbon, C., Young, S.N., Hennessy, P.J., Smith, P.P.C., Webb, A.I., Czabotar, P.E., Koide, S., Murphy, J.M.(2020) Proc Natl Acad Sci U S A 117: 8468-8475
- PubMed: 32234780
- DOI: https://doi.org/10.1073/pnas.1919960117
- Primary Citation of Related Structures:
6UX8 - PubMed Abstract:
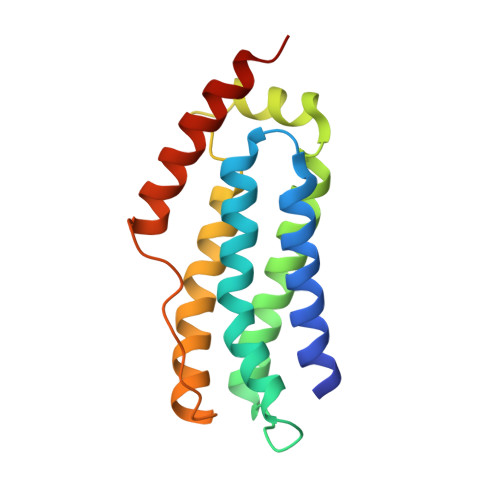
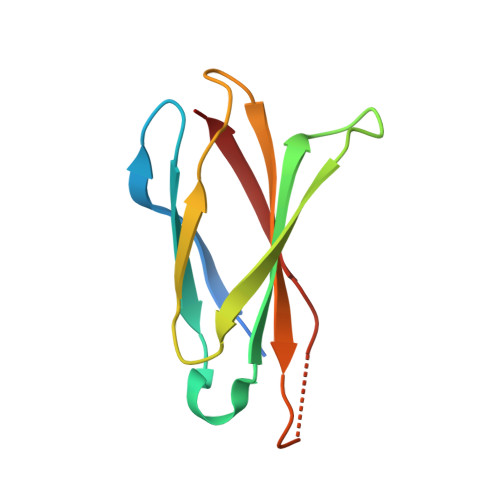
The necroptosis cell death pathway has been implicated in host defense and in the pathology of inflammatory diseases. While phosphorylation of the necroptotic effector pseudokinase Mixed Lineage Kinase Domain-Like (MLKL) by the upstream protein kinase RIPK3 is a hallmark of pathway activation, the precise checkpoints in necroptosis signaling are still unclear. Here we have developed monobodies, synthetic binding proteins, that bind the N-terminal four-helix bundle (4HB) "killer" domain and neighboring first brace helix of human MLKL with nanomolar affinity. When expressed as genetically encoded reagents in cells, these monobodies potently block necroptotic cell death. However, they did not prevent MLKL recruitment to the "necrosome" and phosphorylation by RIPK3, nor the assembly of MLKL into oligomers, but did block MLKL translocation to membranes where activated MLKL normally disrupts membranes to kill cells. An X-ray crystal structure revealed a monobody-binding site centered on the α4 helix of the MLKL 4HB domain, which mutational analyses showed was crucial for reconstitution of necroptosis signaling. These data implicate the α4 helix of its 4HB domain as a crucial site for recruitment of adaptor proteins that mediate membrane translocation, distinct from known phospholipid binding sites.
- Walter and Eliza Hall Institute of Medical Research, Parkville, VIC 3052, Australia.
Organizational Affiliation: