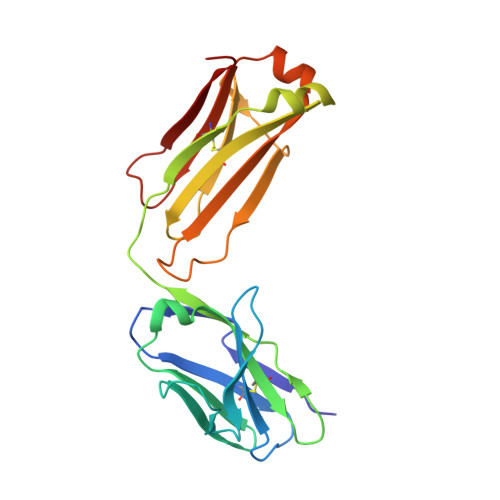
Conformational Flexibility in Respiratory Syncytial Virus G Neutralizing Epitopes.
Fedechkin, S.O., George, N.L., Nunez Castrejon, A.M., Dillen, J.R., Kauvar, L.M., DuBois, R.M.(2020) J Virol 94
- PubMed: 31852779
- DOI: https://doi.org/10.1128/JVI.01879-19
- Primary Citation of Related Structures:
6UVO - PubMed Abstract:
Respiratory syncytial virus (RSV) is a top cause of severe lower respiratory tract disease and mortality in infants and the elderly. Currently, no vaccine or effective treatment exists for RSV. The RSV G glycoprotein mediates viral attachment to cells and contributes to pathogenesis by modulating host immunity through interactions with the human chemokine receptor CX3CR1. Antibodies targeting the RSV G central conserved domain are protective in both prophylactic and postinfection animal models. Here, we describe the crystal structure of the broadly neutralizing human monoclonal antibody 3G12 bound to the RSV G central conserved domain. Antibody 3G12 binds to a conformational epitope composed of highly conserved residues, explaining its broad neutralization activity. Surprisingly, RSV G complexed with 3G12 adopts a distinct conformation not observed in previously described RSV G-antibody structures. Comparison to other structures reveals that the RSV G central conserved domain is flexible and can adopt multiple conformations in the regions flanking the cysteine noose. We also show that restriction of RSV G flexibility with a proline mutation abolishes binding to antibody 3G12 but not antibody 3D3, which recognizes a different conformation of RSV G. Our studies provide new insights for rational vaccine design, indicating the importance of preserving both the global structural integrity of antigens and local conformational flexibility at antigenic sites, which may elicit a more diverse antibody response and broader protection against infection and disease. IMPORTANCE Respiratory syncytial virus (RSV) causes severe respiratory infections in infants, young children, and the elderly, and currently, no licensed vaccine exists. In this study, we describe the crystal structure of the RSV surface glycoprotein G in complex with a broadly neutralizing human monoclonal antibody. The antibody binds to RSV G at a highly conserved region stabilized by two disulfide bonds, but it captures RSV G in a conformation not previously observed, revealing that this region is both structured and flexible. Importantly, our findings provide insight for the design of vaccines that elicit diverse antibodies, which may provide broad protection from infection and disease.
- Department of Biomolecular Engineering, University of California-Santa Cruz, Santa Cruz, California, USA.
Organizational Affiliation: