iAChSnFR Fluorescent Acetylcholine Sensor precursor
Borden, P.M., Marvin, J.S., Looger, L.L.To be published.
Experimental Data Snapshot
Starting Models: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| iAChSnFR precursor | 558 | Escherichia coli | Mutation(s): 0 | 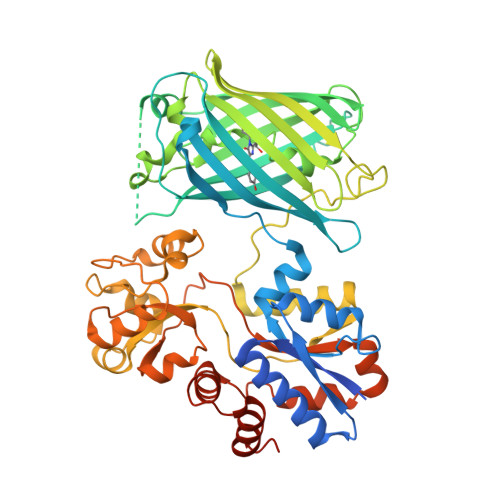 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CRO Query on CRO | A, B | L-PEPTIDE LINKING | C15 H17 N3 O5 |  | THR, TYR, GLY |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 72.12 | α = 90 |
| b = 89.78 | β = 90.48 |
| c = 92.89 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| iMOSFLM | data reduction |
| SCALA | data scaling |
| PHASER | phasing |