Cryo-EM structure of NPF-bound human Arp2/3 complex and activation mechanism.
Zimmet, A., Van Eeuwen, T., Boczkowska, M., Rebowski, G., Murakami, K., Dominguez, R.(2020) Sci Adv 6: eaaz7651-eaaz7651
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2020) Sci Adv 6: eaaz7651-eaaz7651
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin-related protein 3 | 418 | Homo sapiens | Mutation(s): 0 Gene Names: ACTR3, ARP3 | 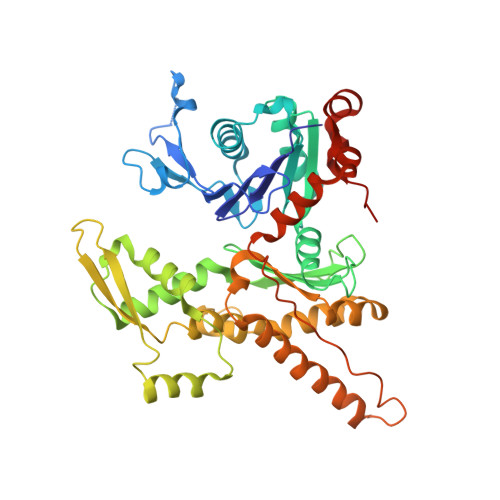 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P61158 (Homo sapiens) Explore P61158 Go to UniProtKB: P61158 | |||||
PHAROS: P61158 GTEx: ENSG00000115091 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P61158 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin-related protein 2 | 394 | Homo sapiens | Mutation(s): 0 Gene Names: ACTR2, ARP2 | 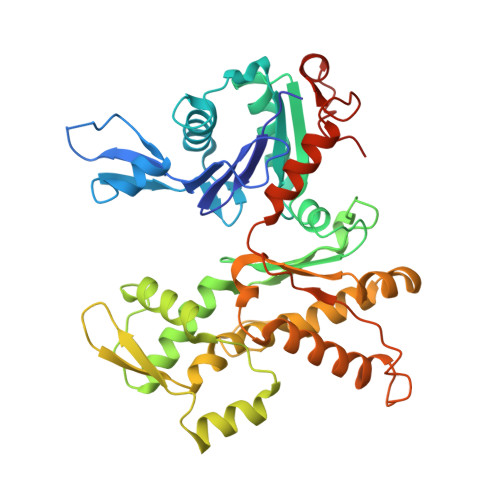 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P61160 (Homo sapiens) Explore P61160 Go to UniProtKB: P61160 | |||||
PHAROS: P61160 GTEx: ENSG00000138071 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P61160 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin-related protein 2/3 complex subunit 1B | 372 | Homo sapiens | Mutation(s): 0 Gene Names: ARPC1B, ARC41 | 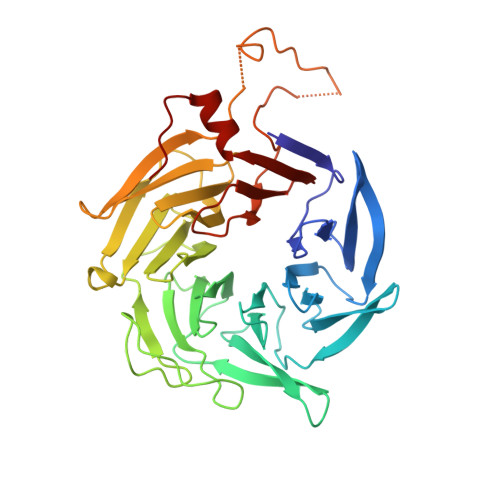 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15143 (Homo sapiens) Explore O15143 Go to UniProtKB: O15143 | |||||
PHAROS: O15143 GTEx: ENSG00000130429 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15143 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin-related protein 2/3 complex subunit 2 | 300 | Homo sapiens | Mutation(s): 0 Gene Names: ARPC2, ARC34, PRO2446 | 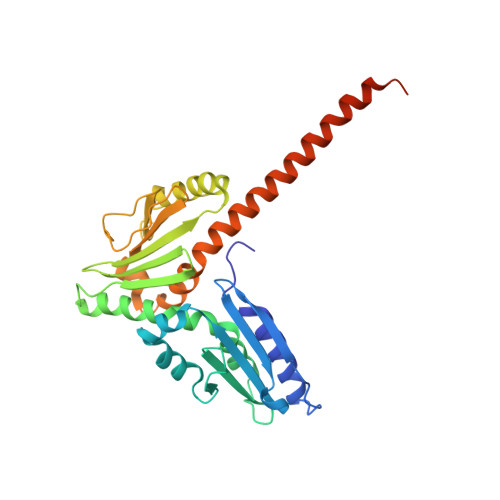 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15144 (Homo sapiens) Explore O15144 Go to UniProtKB: O15144 | |||||
PHAROS: O15144 GTEx: ENSG00000163466 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15144 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 5 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin-related protein 2/3 complex subunit 3 | 203 | Homo sapiens | Mutation(s): 0 Gene Names: ARPC3, ARC21 | 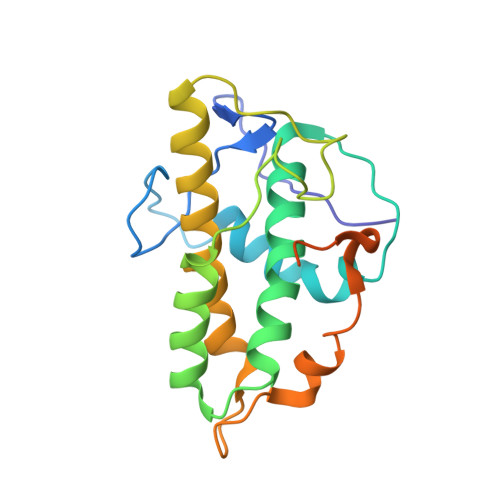 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15145 (Homo sapiens) Explore O15145 Go to UniProtKB: O15145 | |||||
PHAROS: O15145 GTEx: ENSG00000111229 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15145 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 6 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin-related protein 2/3 complex subunit 4 | 168 | Homo sapiens | Mutation(s): 0 Gene Names: ARPC4, ARC20 | 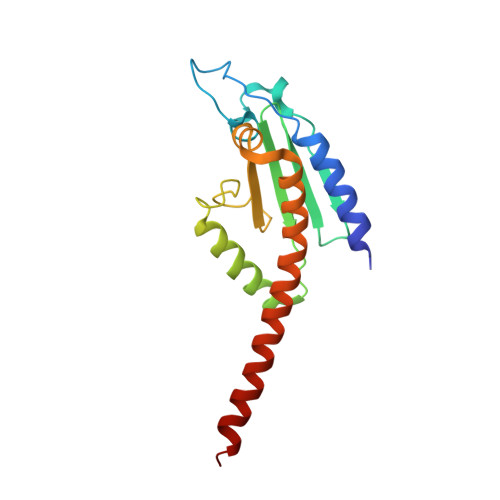 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P59998 (Homo sapiens) Explore P59998 Go to UniProtKB: P59998 | |||||
PHAROS: P59998 GTEx: ENSG00000241553 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P59998 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 7 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin-related protein 2/3 complex subunit 5 | 151 | Homo sapiens | Mutation(s): 0 Gene Names: ARPC5, ARC16 | 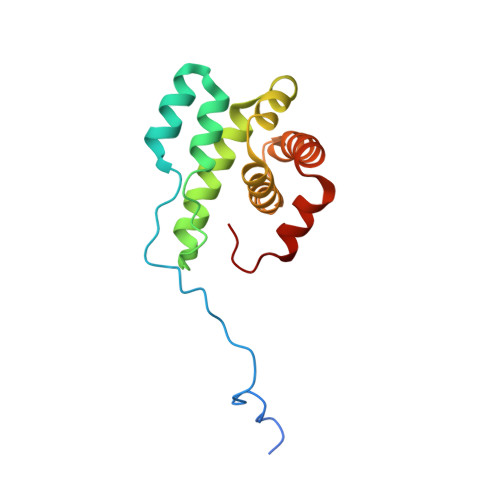 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15511 (Homo sapiens) Explore O15511 Go to UniProtKB: O15511 | |||||
PHAROS: O15511 GTEx: ENSG00000162704 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15511 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 8 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Neural Wiskott-Aldrich syndrome protein | 501 | Mus musculus | Mutation(s): 0 Gene Names: Wasl | 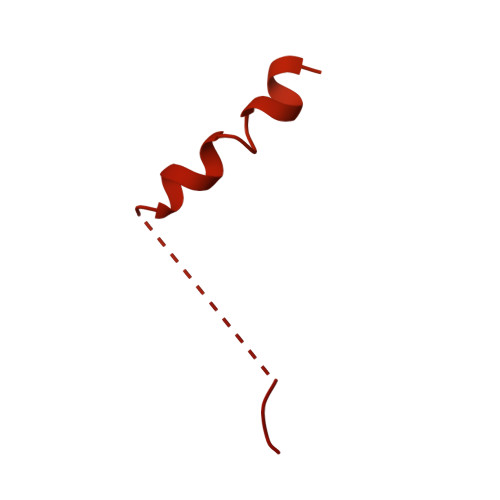 | |
UniProt | |||||
Find proteins for Q91YD9 (Mus musculus) Explore Q91YD9 Go to UniProtKB: Q91YD9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q91YD9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ATP Query on ATP | K [auth A], M [auth B] | ADENOSINE-5'-TRIPHOSPHATE C10 H16 N5 O13 P3 ZKHQWZAMYRWXGA-KQYNXXCUSA-N |  | ||
| MG Query on MG | J [auth A], L [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC | 2.9.0 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R01-GM07391 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | T32-GM008275 |
| National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI) | United States | F31-HL146077 |