Structural basis for CDC42 and RAC activation by the dual specificity GEF DOCK10
Fan, D., Yang, J., Cronin, N., Barford, D.(2022) bioRxiv
Experimental Data Snapshot
Starting Models: experimental
View more details
wwPDB Validation 3D Report Full Report
(2022) bioRxiv
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Dedicator of cytokinesis protein 10 | A [auth B] | 457 | Homo sapiens | Mutation(s): 0 Gene Names: DOCK10, KIAA0694, ZIZ3 | 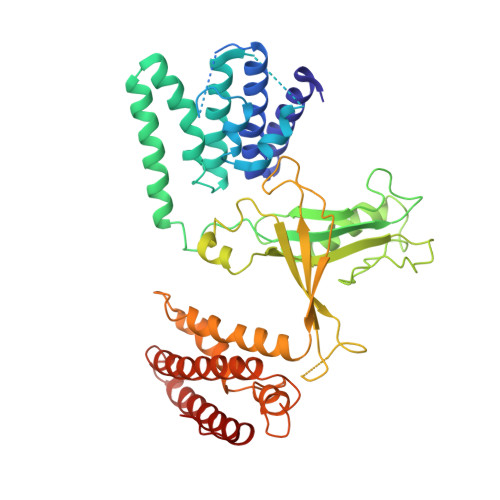 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q96BY6 (Homo sapiens) Explore Q96BY6 Go to UniProtKB: Q96BY6 | |||||
PHAROS: Q96BY6 GTEx: ENSG00000135905 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q96BY6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ras-related C3 botulinum toxin substrate 3 | B [auth A] | 192 | Homo sapiens | Mutation(s): 0 Gene Names: RAC3 EC: 3.6.5.2 | 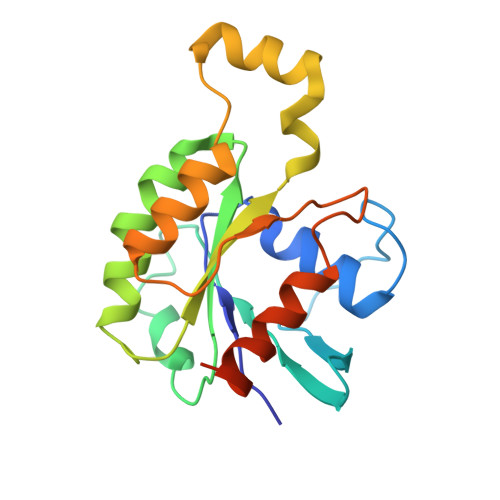 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P60763 (Homo sapiens) Explore P60763 Go to UniProtKB: P60763 | |||||
PHAROS: P60763 GTEx: ENSG00000169750 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P60763 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Dedicator of cytokinesis protein 10 | 458 | Homo sapiens | Mutation(s): 0 Gene Names: DOCK10, KIAA0694, ZIZ3 | 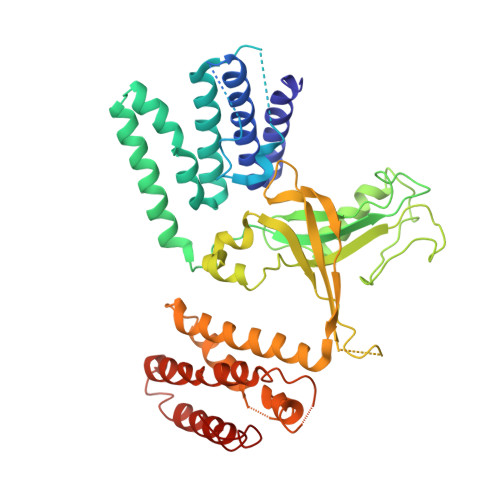 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q96BY6 (Homo sapiens) Explore Q96BY6 Go to UniProtKB: Q96BY6 | |||||
PHAROS: Q96BY6 GTEx: ENSG00000135905 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q96BY6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 110.079 | α = 90 |
| b = 128.455 | β = 90 |
| c = 215.102 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| PHENIX | refinement |
| MOSFLM | data reduction |
| SCALA | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Cancer Research UK | United Kingdom | C576/A14109 |