MUZ
Query on MUZ
Download Ideal Coordinates CCD File
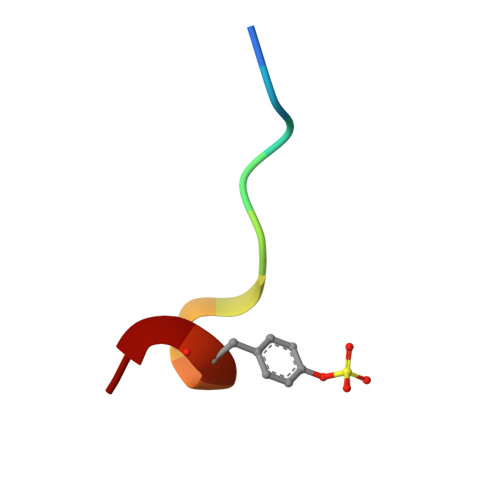
| D [auth H] | [(~{R})-(4-carbamimidoylphenyl)-[[(2~{S})-1-[(2~{R})-3-cyclohexyl-2-[(phenylmethyl)sulfonylamino]propanoyl]pyrrolidin-2-yl]carbonylamino]methyl]-phenoxy-phosphinous acid
C35 H44 N5 O6 P S
ISCJXVGAKGBLBF-MLJJTDPGSA-N |  | |
NAG
Query on NAG
Download Ideal Coordinates CCD File
| E [auth H] | 2-acetamido-2-deoxy-beta-D-glucopyranose
C8 H15 N O6
OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | |
PO4
Query on PO4
Download Ideal Coordinates CCD File
| G [auth H] | PHOSPHATE ION
O4 P
NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | |
DMS
Query on DMS
Download Ideal Coordinates CCD File
| I [auth H],
J [auth H] | DIMETHYL SULFOXIDE
C2 H6 O S
IAZDPXIOMUYVGZ-UHFFFAOYSA-N |  | |
NA
Query on NA
Download Ideal Coordinates CCD File
| F [auth H],
H | SODIUM ION
Na
FKNQFGJONOIPTF-UHFFFAOYSA-N |  | |