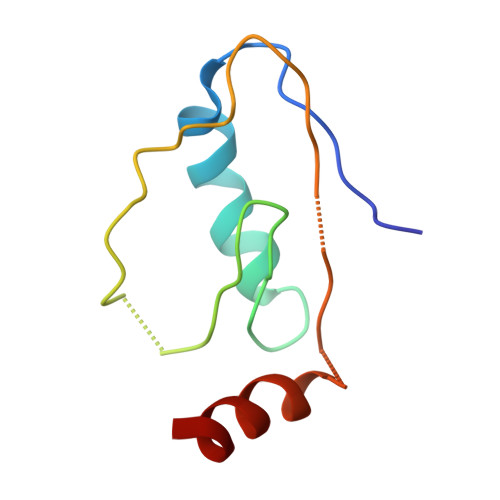
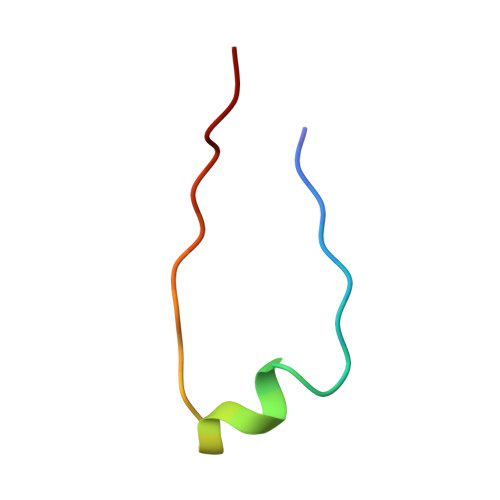
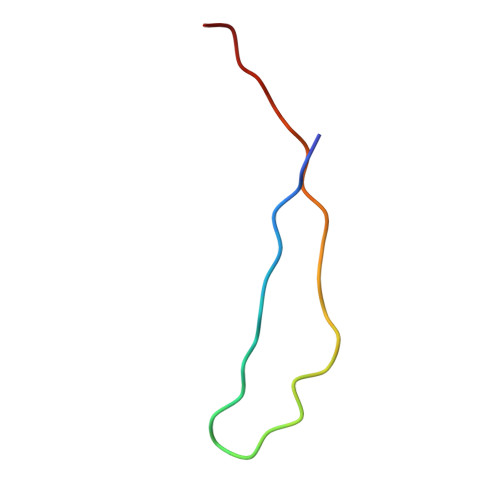
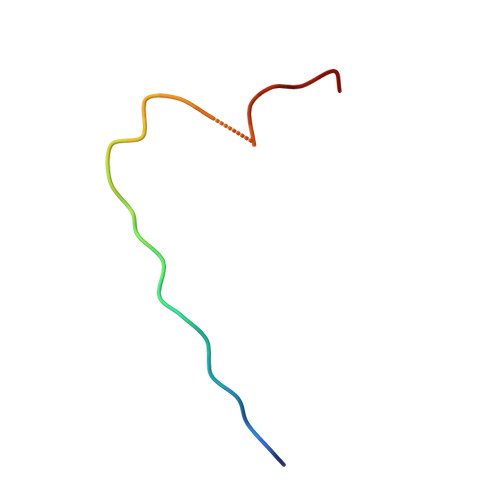
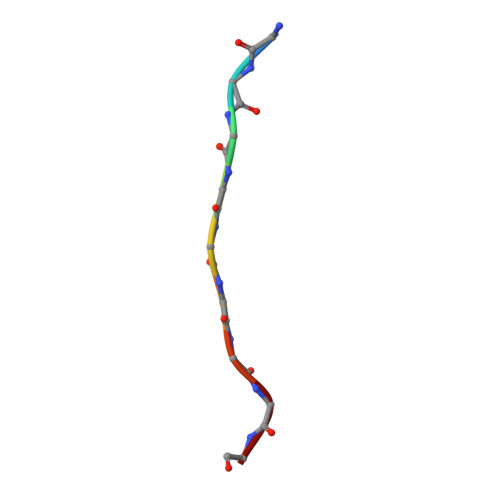
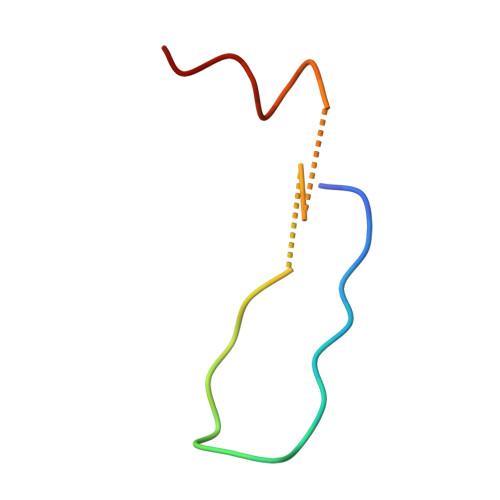
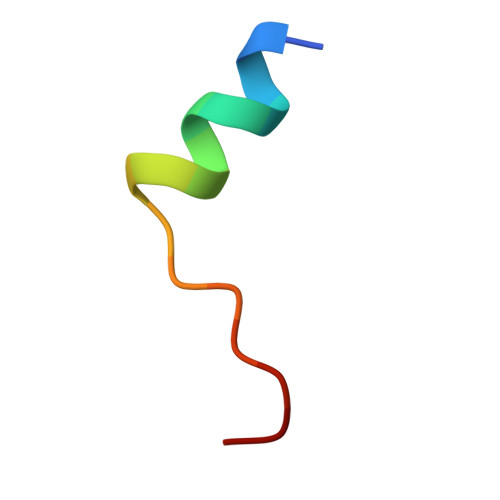
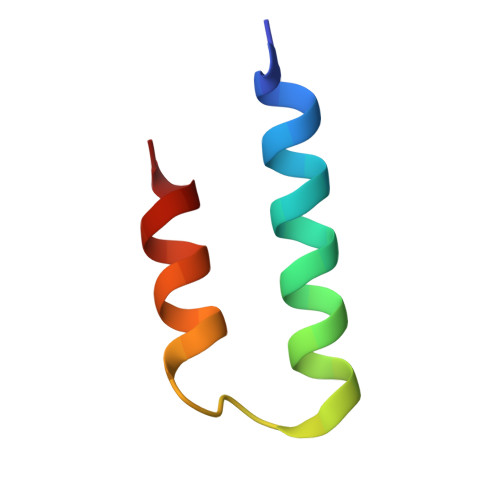
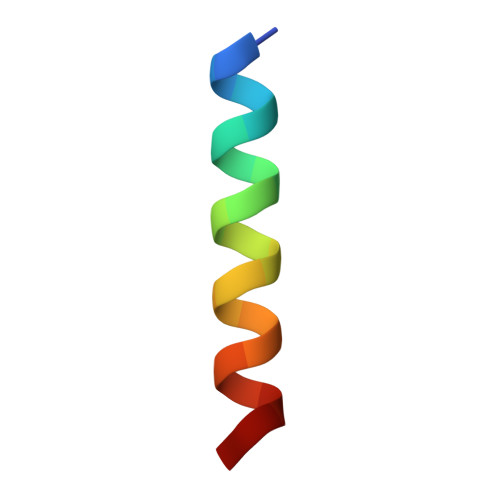
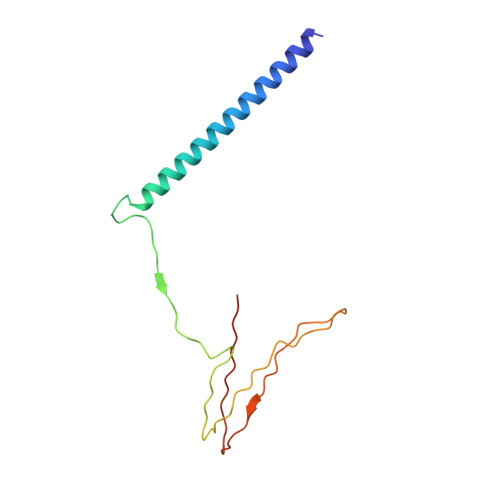
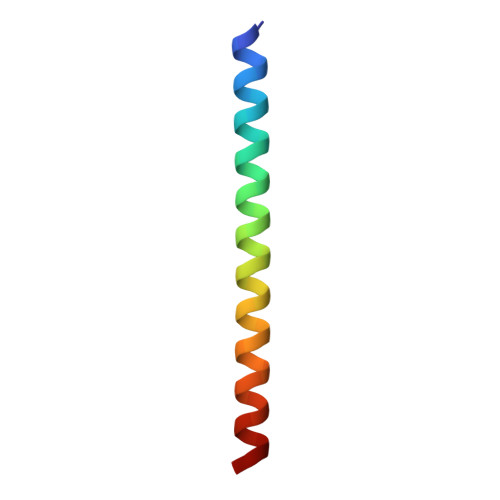
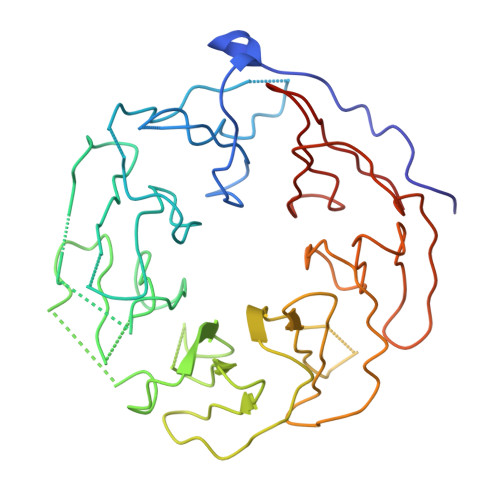
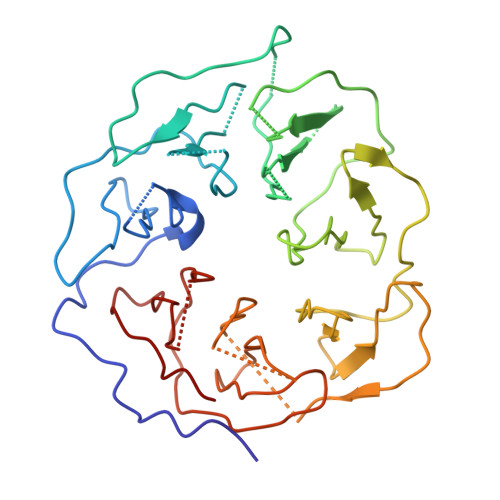
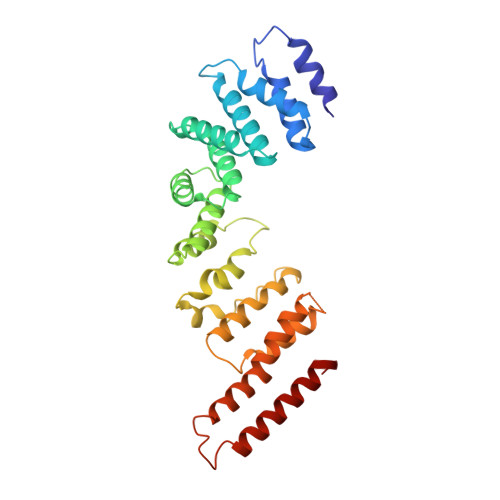
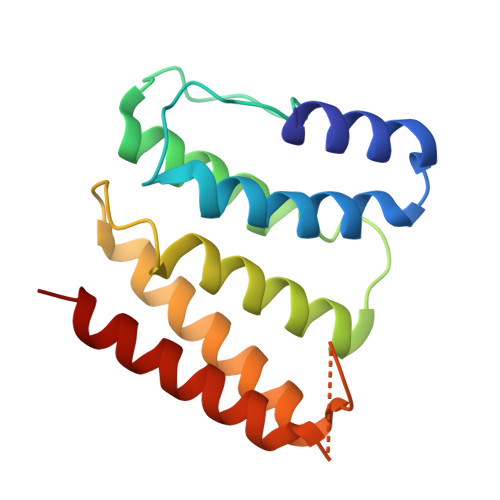
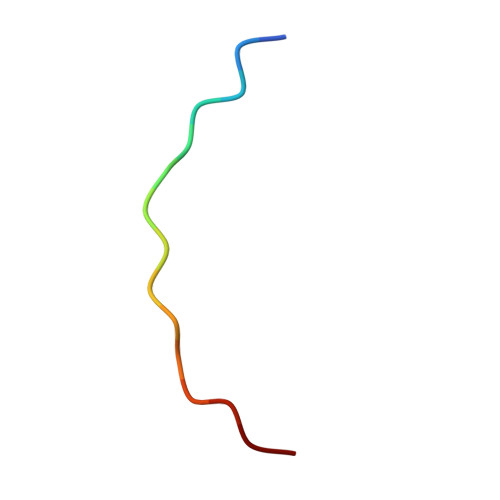Structure of the Fanconi anaemia monoubiquitin ligase complex.
Shakeel, S., Rajendra, E., Alcon, P., O'Reilly, F., Chorev, D.S., Maslen, S., Degliesposti, G., Russo, C.J., He, S., Hill, C.H., Skehel, J.M., Scheres, S.H.W., Patel, K.J., Rappsilber, J., Robinson, C.V., Passmore, L.A.(2019) Nature 575: 234-237
- PubMed: 31666700
- DOI: https://doi.org/10.1038/s41586-019-1703-4
- Primary Citation of Related Structures:
6SRI, 6SRS - PubMed Abstract:
The Fanconi anaemia (FA) pathway repairs DNA damage caused by endogenous and chemotherapy-induced DNA crosslinks, and responds to replication stress 1,2 . Genetic inactivation of this pathway by mutation of genes encoding FA complementation group (FANC) proteins impairs development, prevents blood production and promotes cancer 1,3 . The key molecular step in the FA pathway is the monoubiquitination of a pseudosymmetric heterodimer of FANCD2-FANCI 4,5 by the FA core complex-a megadalton multiprotein E3 ubiquitin ligase 6,7 . Monoubiquitinated FANCD2 then recruits additional protein factors to remove the DNA crosslink or to stabilize the stalled replication fork. A molecular structure of the FA core complex would explain how it acts to maintain genome stability. Here we reconstituted an active, recombinant FA core complex, and used cryo-electron microscopy and mass spectrometry to determine its structure. The FA core complex comprises two central dimers of the FANCB and FA-associated protein of 100 kDa (FAAP100) subunits, flanked by two copies of the RING finger subunit, FANCL. These two heterotrimers act as a scaffold to assemble the remaining five subunits, resulting in an extended asymmetric structure. Destabilization of the scaffold would disrupt the entire complex, resulting in a non-functional FA pathway. Thus, the structure provides a mechanistic basis for the low numbers of patients with mutations in FANCB, FANCL and FAAP100. Despite a lack of sequence homology, FANCB and FAAP100 adopt similar structures. The two FANCL subunits are in different conformations at opposite ends of the complex, suggesting that each FANCL has a distinct role. This structural and functional asymmetry of dimeric RING finger domains may be a general feature of E3 ligases. The cryo-electron microscopy structure of the FA core complex provides a foundation for a detailed understanding of its E3 ubiquitin ligase activity and DNA interstrand crosslink repair.
- MRC Laboratory of Molecular Biology, Cambridge, UK.
Organizational Affiliation: