Strategies for Late-Stage Optimization: Profiling Thermodynamics by Preorganization and Salt Bridge Shielding.
Sandner, A., Hufner-Wulsdorf, T., Heine, A., Steinmetzer, T., Klebe, G.(2019) J Med Chem 62: 9753-9771
- PubMed: 31633354
- DOI: https://doi.org/10.1021/acs.jmedchem.9b01196
- Primary Citation of Related Structures:
5JFD, 5JZY, 5LCE, 5LPD, 6GBW, 6ROT - PubMed Abstract:
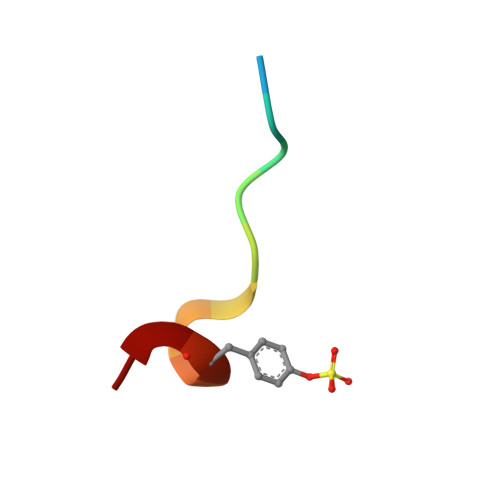
Structural fixation of a ligand in its bioactive conformation may, due to entropic reasons, improve affinity. We present a congeneric series of thrombin ligands with a variety of functional groups triggering preorganization prior to binding. Fixation in solution and complex formation have been characterized by crystallography, isothermal titration calorimetry (ITC), and molecular dynamics (MD) simulations. First, we show why these preorganizing modifications do not affect the overall binding mode and how key interactions are preserved. Next, we demonstrate how preorganization thermodynamics can be largely dominated by enthalpy rather than entropy because of the significant population of low-energy conformations. Furthermore, a salt bridge is shielded by actively reducing its surface exposure, thus leading to an enhanced enthalpic binding profile. Our results suggest that the consideration of the ligand solution ensemble by MD simulation is necessary to predict preorganizing modifications that enhance the binding behavior of already promising binders.
- Institut für Pharmazeutische Chemie , Philipps-Universität Marburg , Marbacher Weg 6 , 35032 Marburg , Germany.
Organizational Affiliation: