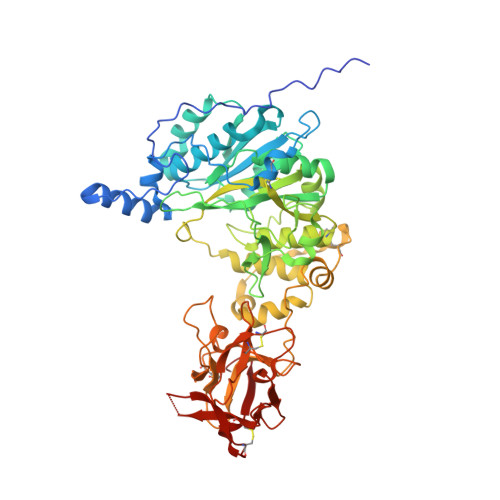
The structure of the colorectal cancer-associated enzyme GalNAc-T12 reveals how nonconserved residues dictate its function.
Fernandez, A.J., Daniel, E.J.P., Mahajan, S.P., Gray, J.J., Gerken, T.A., Tabak, L.A., Samara, N.L.(2019) Proc Natl Acad Sci U S A 116: 20404-20410
- PubMed: 31548401
- DOI: https://doi.org/10.1073/pnas.1902211116
- Primary Citation of Related Structures:
6PXU - PubMed Abstract:
Polypeptide N- acetylgalactosaminyl transferases (GalNAc-Ts) initiate mucin type O -glycosylation by catalyzing the transfer of N -acetylgalactosamine (GalNAc) to Ser or Thr on a protein substrate. Inactive and partially active variants of the isoenzyme GalNAc-T12 are present in subsets of patients with colorectal cancer, and several of these variants alter nonconserved residues with unknown functions. While previous biochemical studies have demonstrated that GalNAc-T12 selects for peptide and glycopeptide substrates through unique interactions with its catalytic and lectin domains, the molecular basis for this distinct substrate selectivity remains elusive. Here we examine the molecular basis of the activity and substrate selectivity of GalNAc-T12. The X-ray crystal structure of GalNAc-T12 in complex with a di-glycosylated peptide substrate reveals how a nonconserved GalNAc binding pocket in the GalNAc-T12 catalytic domain dictates its unique substrate selectivity. In addition, the structure provides insight into how colorectal cancer mutations disrupt the activity of GalNAc-T12 and illustrates how the rules dictating GalNAc-T12 function are distinct from those for other GalNAc-Ts.
- Section on Biological Chemistry, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
Organizational Affiliation: