Computational design of a modular protein sense-response system.
Glasgow, A.A., Huang, Y.M., Mandell, D.J., Thompson, M., Ritterson, R., Loshbaugh, A.L., Pellegrino, J., Krivacic, C., Pache, R.A., Barlow, K.A., Ollikainen, N., Jeon, D., Kelly, M.J.S., Fraser, J.S., Kortemme, T.(2019) Science 366: 1024-1028
- PubMed: 31754004
- DOI: https://doi.org/10.1126/science.aax8780
- Primary Citation of Related Structures:
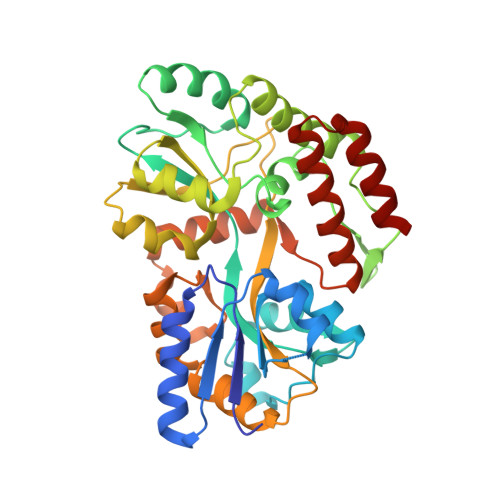
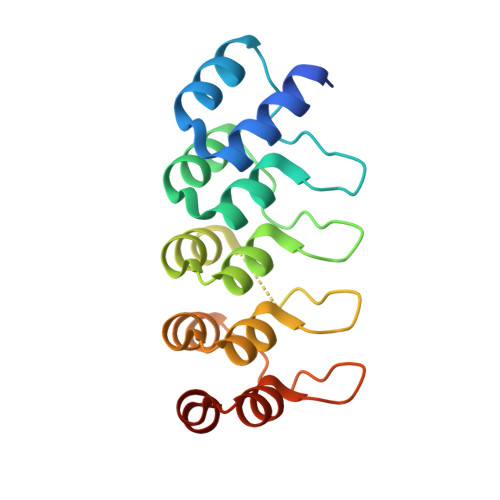
6OB5 - PubMed Abstract:
Sensing and responding to signals is a fundamental ability of living systems, but despite substantial progress in the computational design of new protein structures, there is no general approach for engineering arbitrary new protein sensors. Here, we describe a generalizable computational strategy for designing sensor-actuator proteins by building binding sites de novo into heterodimeric protein-protein interfaces and coupling ligand sensing to modular actuation through split reporters. Using this approach, we designed protein sensors that respond to farnesyl pyrophosphate, a metabolic intermediate in the production of valuable compounds. The sensors are functional in vitro and in cells, and the crystal structure of the engineered binding site closely matches the design model. Our computational design strategy opens broad avenues to link biological outputs to new signals.
- Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, CA, USA.
Organizational Affiliation: