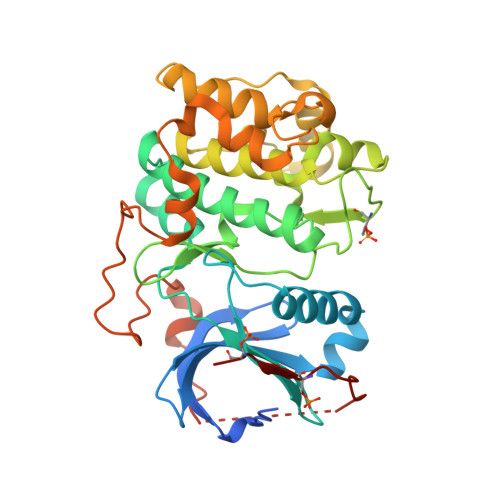
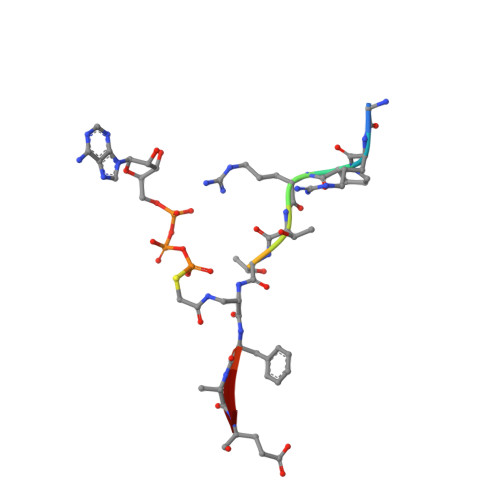
Akt Kinase Activation Mechanisms Revealed Using Protein Semisynthesis.
Chu, N., Salguero, A.L., Liu, A.Z., Chen, Z., Dempsey, D.R., Ficarro, S.B., Alexander, W.M., Marto, J.A., Li, Y., Amzel, L.M., Gabelli, S.B., Cole, P.A.(2018) Cell 174: 897-907.e14
- PubMed: 30078705
- DOI: https://doi.org/10.1016/j.cell.2018.07.003
- Primary Citation of Related Structures:
6BUU, 6NPZ - PubMed Abstract:
Akt is a critical protein kinase that drives cancer proliferation, modulates metabolism, and is activated by C-terminal phosphorylation. The current structural model for Akt activation by C-terminal phosphorylation has centered on intramolecular interactions between the C-terminal tail and the N lobe of the kinase domain. Here, we employ expressed protein ligation to produce site-specifically phosphorylated forms of purified Akt1 that are well suited for mechanistic analysis. Using biochemical, crystallographic, and cellular approaches, we determine that pSer473-Akt activation is driven by an intramolecular interaction between the C-tail and the pleckstrin homology (PH)-kinase domain linker that relieves PH domain-mediated Akt1 autoinhibition. Moreover, dual phosphorylation at Ser477/Thr479 activates Akt1 through a different allosteric mechanism via an apparent activation loop interaction that reduces autoinhibition by the PH domain and weakens PIP3 affinity. These results provide a new framework for understanding how Akt is controlled in cell signaling and suggest distinct functions for differentially modified Akt forms.
- Division of Genetics, Department of Medicine, Brigham and Women's Hospital, Boston, MA 02115, USA; Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA; Department of Pharmacology and Molecular Sciences, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA.
Organizational Affiliation: