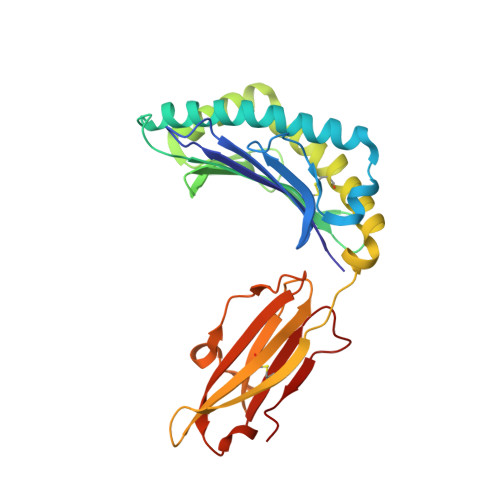
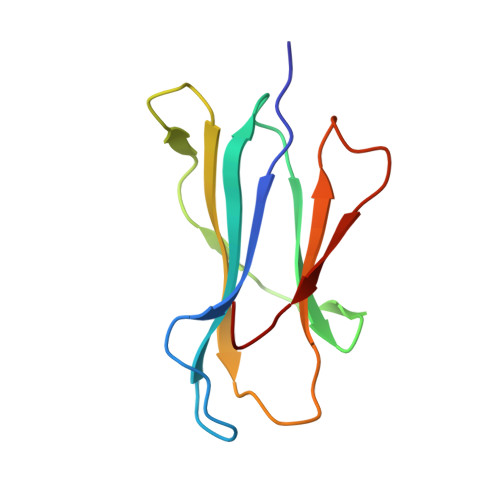
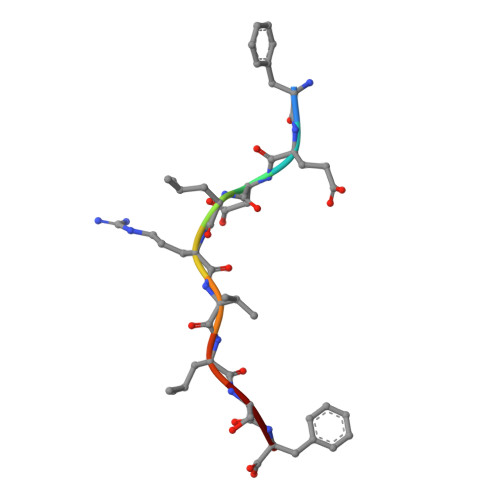
Broad CD8+T cell cross-recognition of distinct influenza A strains in humans.
Grant, E.J., Josephs, T.M., Loh, L., Clemens, E.B., Sant, S., Bharadwaj, M., Chen, W., Rossjohn, J., Gras, S., Kedzierska, K.(2018) Nat Commun 9: 5427-5427
- PubMed: 30575715
- DOI: https://doi.org/10.1038/s41467-018-07815-5
- Primary Citation of Related Structures:
6MT3, 6MT4, 6MT5, 6MT6, 6MTL, 6MTM - PubMed Abstract:
Newly-emerged and vaccine-mismatched influenza A viruses (IAVs) result in a rapid global spread of the virus due to minimal antibody-mediated immunity. In that case, established CD8 + T-cells can reduce disease severity. However, as mutations occur sporadically within immunogenic IAV-derived T-cell peptides, understanding of T-cell receptor (TCRαβ) cross-reactivity towards IAV variants is needed for a vaccine design. Here, we investigate TCRαβ cross-strain recognition across IAV variants within two immunodominant human IAV-specific CD8 + T-cell epitopes, HLA-B*37:01-restricted NP 338-346 (B37-NP 338 ) and HLA-A*01:01-restricted NP 44-52 (A1-NP 44 ). We find high abundance of cross-reactive TCRαβ clonotypes recognizing distinct IAV variants. Structures of the wild-type and variant peptides revealed preserved conformation of the bound peptides. Structures of a cross-reactive TCR-HLA-B37-NP 338 complex suggest that the conserved conformation of the variants underpins TCR cross-reactivity. Overall, cross-reactive CD8 + T-cell responses, underpinned by conserved epitope structure, facilitates recognition of distinct IAV variants, thus CD8 + T-cell-targeted vaccines could provide protection across different IAV strains.
- Department of Microbiology and Immunology, The University of Melbourne, at the Peter Doherty Institute for Infection and Immunity, Melbourne, VIC, 3010, Australia.
Organizational Affiliation: