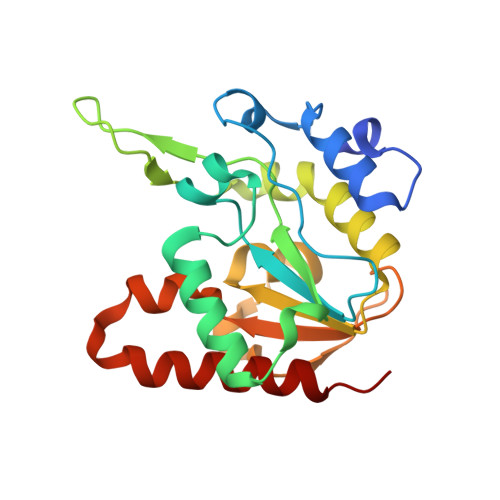
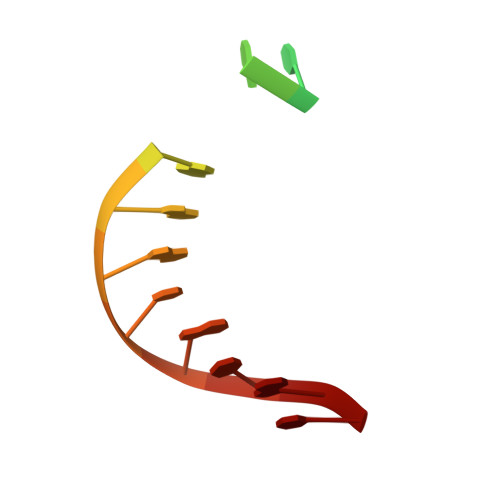
Structural insights into an MsmUdgX mutant capable of both crosslinking and uracil excision capability.
Jia, Q., Zeng, H., Tu, J., Sun, L., Cao, W., Xie, W.(2021) DNA Repair (Amst) 97: 103008-103008
- PubMed: 33248387
- DOI: https://doi.org/10.1016/j.dnarep.2020.103008
- Primary Citation of Related Structures:
6L5A, 6L5B, 6L6S - PubMed Abstract:
UdgX from Mycobacterium smegmatis (MsmUdgX) is a prototypical enzyme representing a new class of uracil-DNA glycosylases (UDG) closely related to the family 4 enzymes. It possesses a unique R-loop rich in positive residues and forms a covalent bond with single-stranded uracil-containing DNAs (ssDNA-Us) that is resistant to denaturants after the removal of the target uracil. We previously identified the H109E mutant of MsmUdgX that forms a weak covalent complex with ssDNA-U and yet possesses moderate uracil excision activity, but the mechanism of its action is not fully understood. To further study the catalytic process of MsmUdgX, we solved the high-resolution crystal structures of H109E in the free and DNA-bound forms, respectively. We found that the key residue Glu109 adopts a similar conformation to that of WT to form the covalent bond, suggesting that it still employs the same "excision-inhibition" mechanism to that of the WT enzyme. The enzyme remains nearly intact before and after the crosslinking reaction, but the first half of the R-loop exhibits large structural differences while the rest of the loop barely moves, owing to the salt-bridge interaction formed via Arg107. Additionally, Arg107, along with Gln53 was found to play important roles in the biochemical properties of MsmUdgX. Our studies provide new insights into the MsmUdgX catalysis and improve our understanding on this unique enzyme.
- MOE Key Laboratory of Gene Function and Regulation, State Key Laboratory for Biocontrol, School of Life Sciences, The Sun Yat-Sen University, Guangzhou, Guangdong, 510006, China.
Organizational Affiliation: