Structural basis for DNA unwinding at forked dsDNA by two coordinating Pif1 helicases.
Su, N., Byrd, A.K., Bharath, S.R., Yang, O., Jia, Y., Tang, X., Ha, T., Raney, K.D., Song, H.(2019) Nat Commun 10: 5375-5375
- PubMed: 31772234
- DOI: https://doi.org/10.1038/s41467-019-13414-9
- Primary Citation of Related Structures:
6L3G - PubMed Abstract:
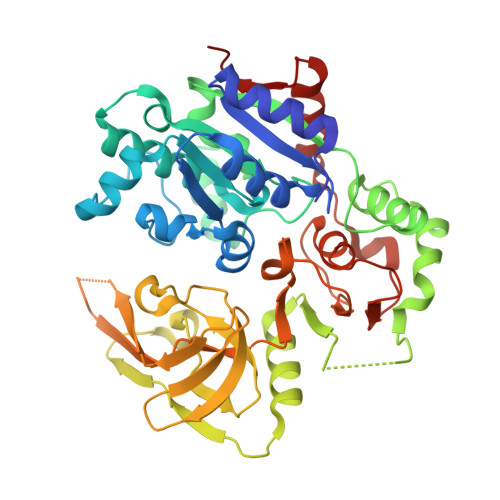
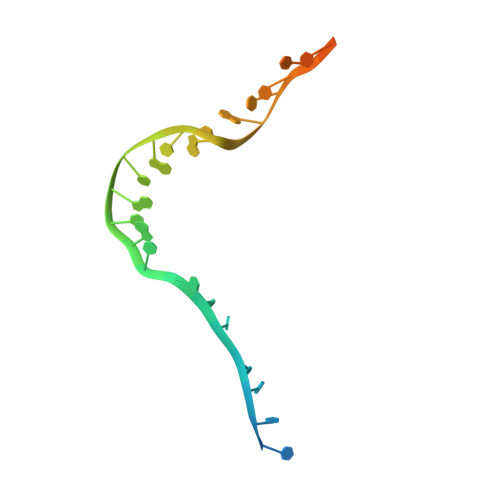
Pif1 plays multiple roles in maintaining genome stability and preferentially unwinds forked dsDNA, but the mechanism by which Pif1 unwinds forked dsDNA remains elusive. Here we report the structure of Bacteroides sp Pif1 (BaPif1) in complex with a symmetrical double forked dsDNA. Two interacting BaPif1 molecules are bound to each fork of the partially unwound dsDNA, and interact with the 5' arm and 3' ss/dsDNA respectively. Each of the two BaPif1 molecules is an active helicase and their interaction may regulate their helicase activities. The binding of BaPif1 to the 5' arm causes a sharp bend in the 5' ss/dsDNA junction, consequently breaking the first base-pair. BaPif1 bound to the 3' ss/dsDNA junction impacts duplex unwinding by stabilizing the unpaired first base-pair and engaging the second base-pair poised for breaking. Our results provide an unprecedented insight into how two BaPif1 coordinate with each other to unwind the forked dsDNA.
- Life Sciences Institute, Zhejiang University, 388 Yuhangtang Road, Hangzhou, 310058, China.
Organizational Affiliation: