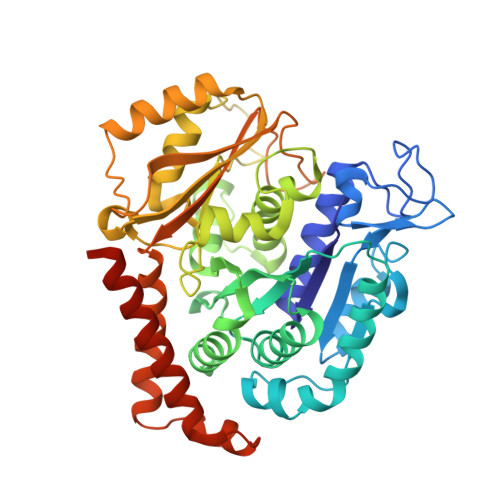
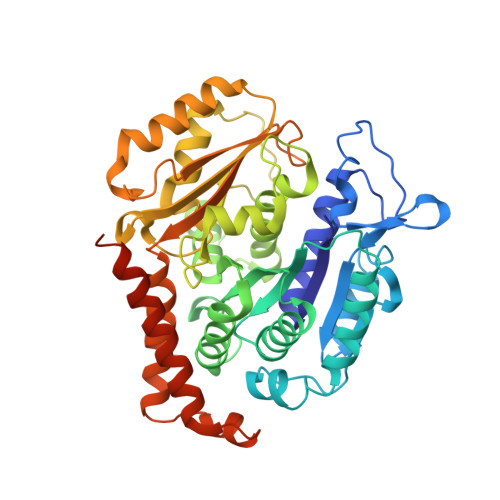
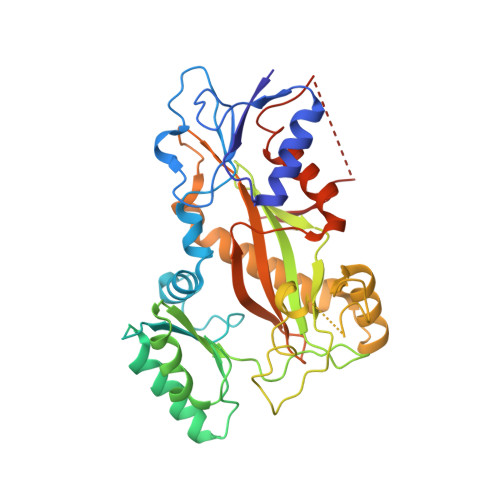
Structural insights into the design of indole derivatives as tubulin polymerization inhibitors.
Li, Y., Yang, J., Niu, L., Hu, D., Li, H., Chen, L., Yu, Y., Chen, Q.(2020) FEBS Lett 594: 199-204
- PubMed: 31369682
- DOI: https://doi.org/10.1002/1873-3468.13566
- Primary Citation of Related Structures:
6K9V - PubMed Abstract:
Microtubules are composed of αβ-tubulin heterodimers, and drugs that interfere with microtubule dynamics are used widely in cancer chemotherapy. Small synthetic molecules with an indole nucleus as a core structure have been identified as microtubule inhibitors and recognized as anticancer agents. However, structural information for the interactions between indole derivatives and tubulin is sparse. Here, we present the 2.55 Å crystal structure of tubulin in complex with the indole derivative D64131. We compare the binding modes of D64131, colchicine, and five other indole derivatives to tubulin. These results reveal the interactions between the indole derivatives and tubulin, explain previous results of structure-activity-relationship (SAR) studies and, thus, provide insights into the development of new indole derivatives targeting the colchicine binding site.
- Cancer Center, West China Hospital, Sichuan University, and Collaborative Innovation Center of Biotherapy, Chengdu, China.
Organizational Affiliation: