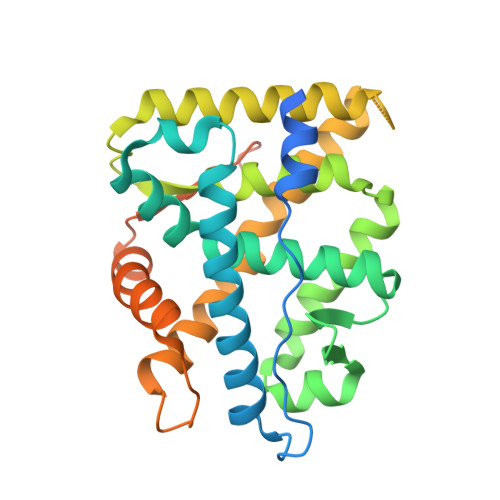
Identification of Mineralocorticoid Receptor Modulators with Low Impact on Electrolyte Homeostasis but Maintained Organ Protection.
Granberg, K.L., Yuan, Z.Q., Lindmark, B., Edman, K., Kajanus, J., Hogner, A., Malmgren, M., O'Mahony, G., Nordqvist, A., Lindberg, J., Tangefjord, S., Kossenjans, M., Lofberg, C., Branalt, J., Liu, D., Selmi, N., Nikitidis, G., Nordberg, P., Hayen, A., Aagaard, A., Hansson, E., Hermansson, M., Ivarsson, I., Jansson-Lofmark, R., Karlsson, U., Johansson, U., William-Olsson, L., Hartleib-Geschwindner, J., Bamberg, K.(2019) J Med Chem 62: 1385-1406
- PubMed: 30596500
- DOI: https://doi.org/10.1021/acs.jmedchem.8b01523
- Primary Citation of Related Structures:
6GEV, 6GG8, 6GGG - PubMed Abstract:
The mechanism-based risk for hyperkalemia has limited the use of mineralocorticoid receptor antagonists (MRAs) like eplerenone in cardio-renal diseases. Here, we describe the structure and property-driven lead generation and optimization, which resulted in identification of MR modulators ( S)-1 and ( S)-33. Both compounds were partial MRAs but still demonstrated equally efficacious organ protection as eplerenone after 4 weeks of treatment in uni-nephrectomized rats on high-salt diet and aldosterone infusion. Importantly, and in sharp contrast to eplerenone, this was achieved without substantial changes to the urine Na + /K + ratio after acute treatment in rat, which predicts a reduced risk for hyperkalemia. This work led to selection of ( S)-1 (AZD9977) as the clinical candidate for treating MR-mediated cardio-renal diseases, including chronic kidney disease and heart failure. On the basis of our findings, we propose an empirical model for prediction of compounds with low risk of affecting the urinary Na + /K + ratio in vivo.
- Pharmaron Beijing Co., Ltd. , No. 6 Taihe Road, BDA , Beijing 100176 , P. R. China.
Organizational Affiliation: