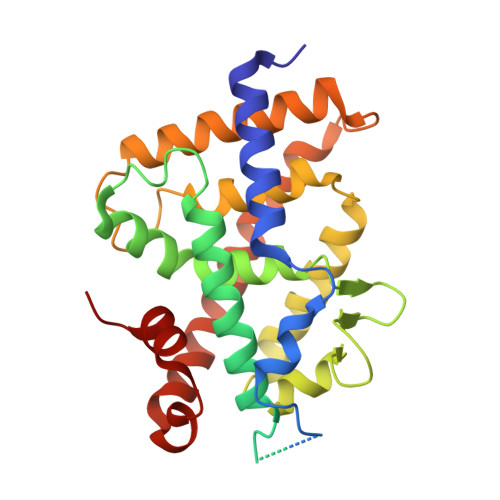
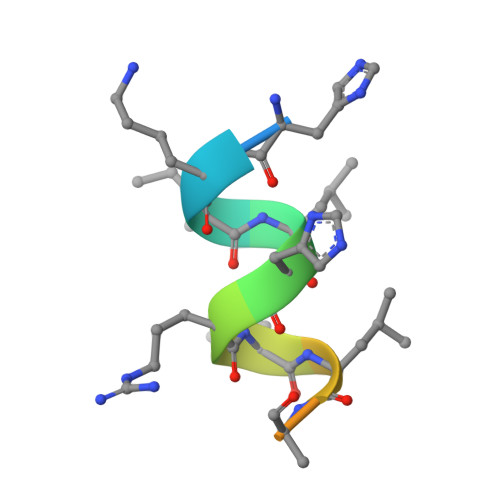
Aromatic-Based Design of Highly Active and Noncalcemic Vitamin D Receptor Agonists.
Gogoi, P., Seoane, S., Sigueiro, R., Guiberteau, T., Maestro, M.A., Perez-Fernandez, R., Rochel, N., Mourino, A.(2018) J Med Chem 61: 4928-4937
- PubMed: 29733645
- DOI: https://doi.org/10.1021/acs.jmedchem.8b00337
- Primary Citation of Related Structures:
6FO7, 6FO8, 6FO9, 6FOB, 6FOD - PubMed Abstract:
We report the design, synthesis, biological evaluation, and structural analysis of a new class of vitamin D analogues that possess an aromatic m-phenylene D-ring and an alkyl chain replacing the C-ring. A key feature of the synthetic strategy is a stereoselective Pd-catalyzed construction of the triene system in aqueous medium that allows the rapid preparation of small amounts of VDR ligands for biological screening. Analogues with the shorter (2a) and longer (2d, 2e) side chains attached to the triene system have no calcemic activity. Compound 2a binds to VDR with the same order of magnitude than calcipotriol and oxacalcitriol. It also reduces proliferation in normal and tumor cells similarly to the natural hormone 1α,25-dihydroxyvitamin D 3 , calcipotriol, and oxacalcitriol, suggesting preclinical studies related to hyperproliferative disorders such as psoriasis and cancer.
- Department of Organic Chemistry, Research Laboratory Ignacio Ribas , University of Santiago de Compostela , Avenida das Ciencias s/n , 15782 Santiago de Compostela , Spain.
Organizational Affiliation: