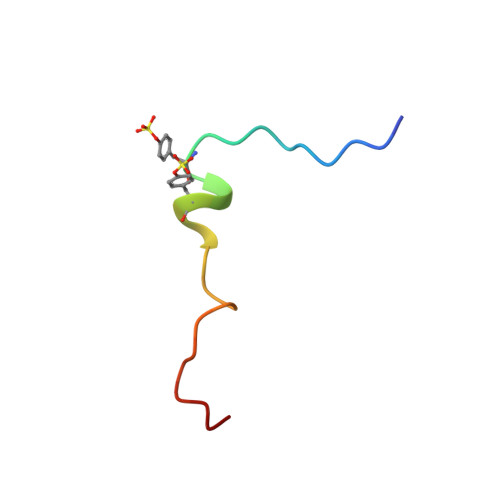
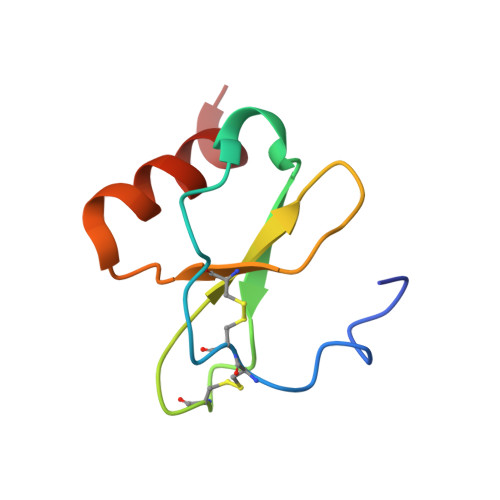
The solution structure of monomeric CCL5 in complex with a doubly sulfated N-terminal segment of CCR5.
Abayev, M., Rodrigues, J.P.G.L.M., Srivastava, G., Arshava, B., Jaremko, L., Jaremko, M., Naider, F., Levitt, M., Anglister, J.(2018) FEBS J 285: 1988-2003
- PubMed: 29619777
- DOI: https://doi.org/10.1111/febs.14460
- Primary Citation of Related Structures:
6FGP - PubMed Abstract:
The inflammatory chemokine CCL5, which binds the chemokine receptor CCR5 in a two-step mechanism so as to activate signaling pathways in hematopoetic cells, plays an important role in immune surveillance, inflammation, and development as well as in several immune system pathologies. The recently published crystal structure of CCR5 bound to a high-affinity variant of CCL5 lacks the N-terminal segment of the receptor that is post-translationally sulfated and is known to be important for high-affinity binding. Here, we report the NMR solution structure of monomeric CCL5 bound to a synthetic doubly sulfated peptide corresponding to the missing first 27 residues of CCR5. Our structures show that two sulfated tyrosine residues, sY10 and sY14, as well as the unsulfated Y15 form a network of strong interactions with a groove on a surface of CCL5 that is formed from evolutionarily conserved basic and hydrophobic amino acids. We then use our NMR structures, in combination with available crystal data, to create an atomic model of full-length wild-type CCR5:CCL5. Our findings reveal the structural determinants involved in the recognition of CCL5 by the CCR5 N terminus. These findings, together with existing structural data, provide a complete structural framework with which to understand the specificity of receptor:chemokine interactions. Structural data are available in the PDB under the accession number 6FGP.
- Department of Structural Biology, Weizmann Institute of Science, Rehovot, Israel.
Organizational Affiliation: