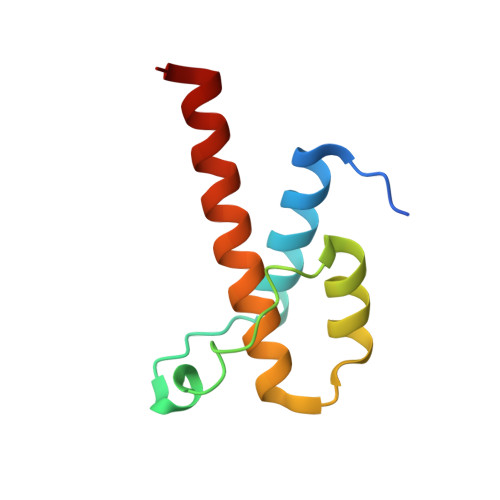
Clathrin light chain A drives selective myosin VI recruitment to clathrin-coated pits under membrane tension.
Biancospino, M., Buel, G.R., Nino, C.A., Maspero, E., Scotto di Perrotolo, R., Raimondi, A., Redlingshofer, L., Weber, J., Brodsky, F.M., Walters, K.J., Polo, S.(2019) Nat Commun 10: 4974-4974
- PubMed: 31672988
- DOI: https://doi.org/10.1038/s41467-019-12855-6
- Primary Citation of Related Structures:
6E5N - PubMed Abstract:
Clathrin light chains (CLCa and CLCb) are major constituents of clathrin-coated vesicles. Unique functions for these evolutionary conserved paralogs remain elusive, and their role in clathrin-mediated endocytosis in mammalian cells is debated. Here, we find and structurally characterize a direct and selective interaction between CLCa and the long isoform of the actin motor protein myosin VI, which is expressed exclusively in highly polarized tissues. Using genetically-reconstituted Caco-2 cysts as proxy for polarized epithelia, we provide evidence for coordinated action of myosin VI and CLCa at the apical surface where these proteins are essential for fission of clathrin-coated pits. We further find that myosin VI and Huntingtin-interacting protein 1-related protein (Hip1R) are mutually exclusive interactors with CLCa, and suggest a model for the sequential function of myosin VI and Hip1R in actin-mediated clathrin-coated vesicle budding.
- IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, 20139, Milan, Italy.
Organizational Affiliation: