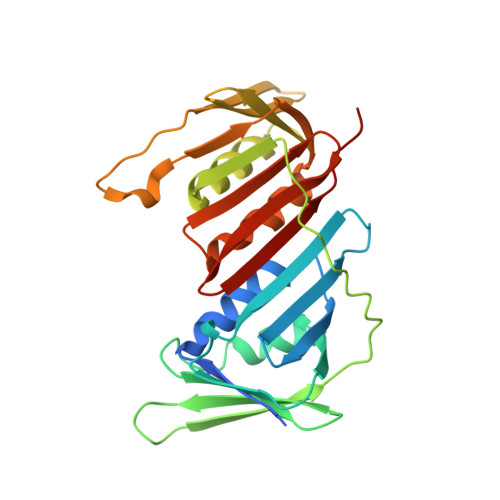
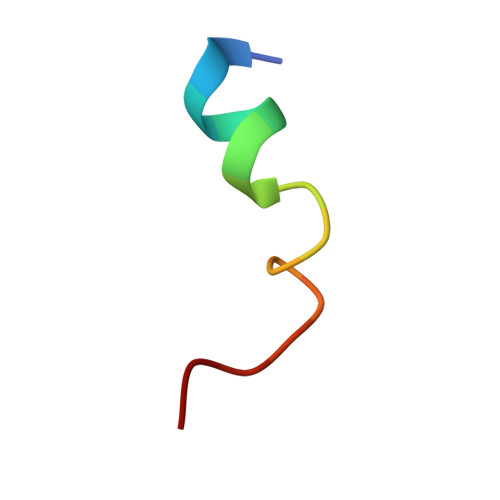
Role of the Pif1-PCNA Complex in Pol delta-Dependent Strand Displacement DNA Synthesis and Break-Induced Replication.
Buzovetsky, O., Kwon, Y., Pham, N.T., Kim, C., Ira, G., Sung, P., Xiong, Y.(2017) Cell Rep 21: 1707-1714
- PubMed: 29141206
- DOI: https://doi.org/10.1016/j.celrep.2017.10.079
- Primary Citation of Related Structures:
6E49 - PubMed Abstract:
The S. cerevisiae Pif1 helicase functions with DNA polymerase (Pol) δ in DNA synthesis during break-induced replication (BIR), a conserved pathway responsible for replication fork repair and telomere recombination. Pif1 interacts with the DNA polymerase processivity clamp PCNA, but the functional significance of the Pif1-PCNA complex remains to be elucidated. Here, we solve the crystal structure of PCNA in complex with a non-canonical PCNA-interacting motif in Pif1. The structure guides the construction of a Pif1 mutant that is deficient in PCNA interaction. This mutation impairs the ability of Pif1 to enhance DNA strand displacement synthesis by Pol δ in vitro and also the efficiency of BIR in cells. These results provide insights into the role of the Pif1-PCNA-Pol δ ensemble during DNA break repair by homologous recombination.
- Department of Molecular Biophysics and Biochemistry, Yale University School of Medicine, New Haven, CT 06520, USA.
Organizational Affiliation: