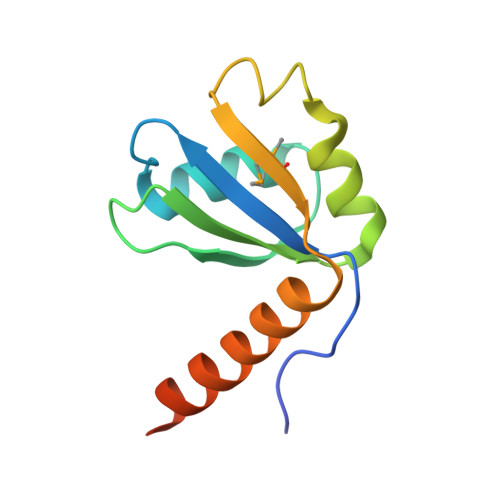
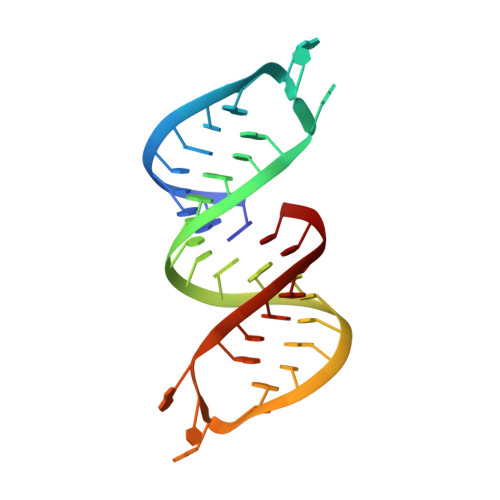
Structural basis for recognition of human 7SK long noncoding RNA by the La-related protein Larp7.
Eichhorn, C.D., Yang, Y., Repeta, L., Feigon, J.(2018) Proc Natl Acad Sci U S A 115: E6457-E6466
- PubMed: 29946027
- DOI: https://doi.org/10.1073/pnas.1806276115
- Primary Citation of Related Structures:
6D12 - PubMed Abstract:
The La and the La-related protein (LARP) superfamily is a diverse class of RNA binding proteins involved in RNA processing, folding, and function. Larp7 binds to the abundant long noncoding 7SK RNA and is required for 7SK ribonucleoprotein (RNP) assembly and function. The 7SK RNP sequesters a pool of the positive transcription elongation factor b (P-TEFb) in an inactive state; on release, P-TEFb phosphorylates RNA Polymerase II to stimulate transcription elongation. Despite its essential role in transcription, limited structural information is available for the 7SK RNP, particularly for protein-RNA interactions. Larp7 contains an N-terminal La module that binds UUU-3'OH and a C-terminal atypical RNA recognition motif (xRRM) required for specific binding to 7SK and P-TEFb assembly. Deletion of the xRRM is linked to gastric cancer in humans. We report the 2.2-Å X-ray crystal structure of the human La-related protein group 7 (hLarp7) xRRM bound to the 7SK stem-loop 4, revealing a unique binding interface. Contributions of observed interactions to binding affinity were investigated by mutagenesis and isothermal titration calorimetry. NMR 13 C spin relaxation data and comparison of free xRRM, RNA, and xRRM-RNA structures show that the xRRM is preordered to bind a flexible loop 4. Combining structures of the hLarp7 La module and the xRRM-7SK complex presented here, we propose a structural model for Larp7 binding to the 7SK 3' end and mechanism for 7SK RNP assembly. This work provides insight into how this domain contributes to 7SK recognition and assembly of the core 7SK RNP.
- Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095-1569.
Organizational Affiliation: