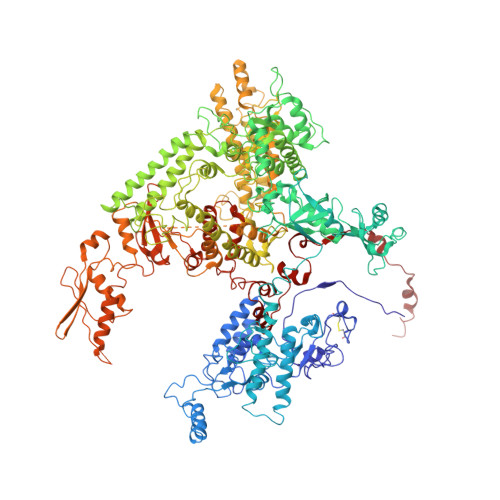
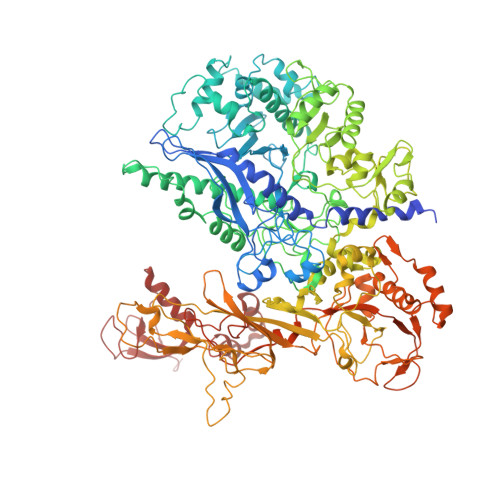
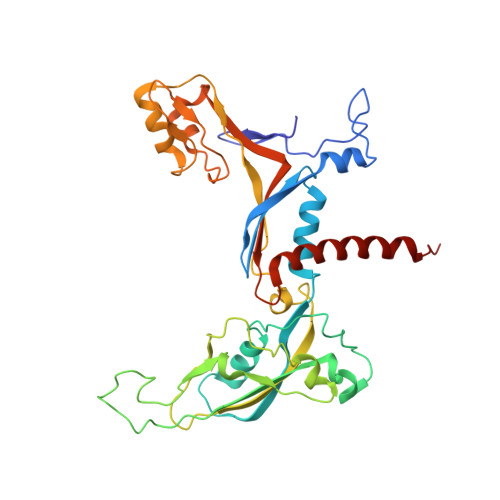
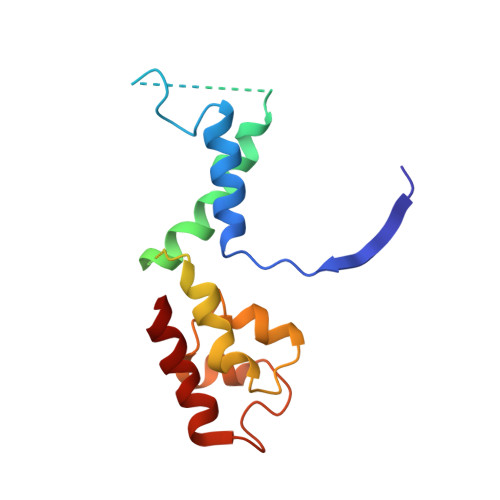
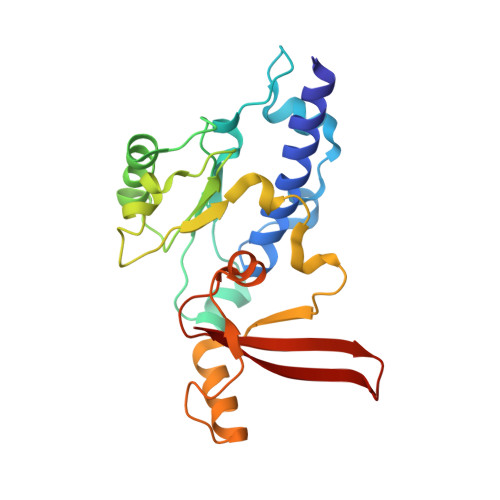
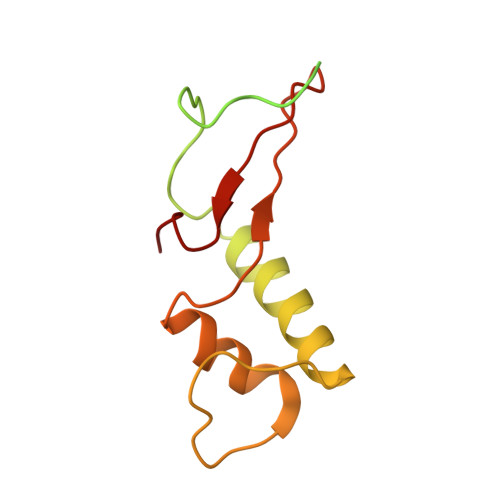
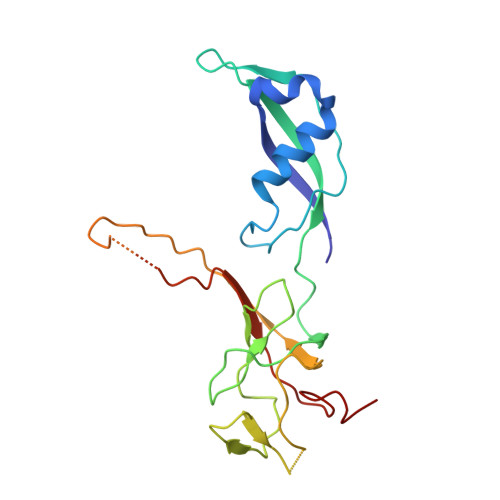
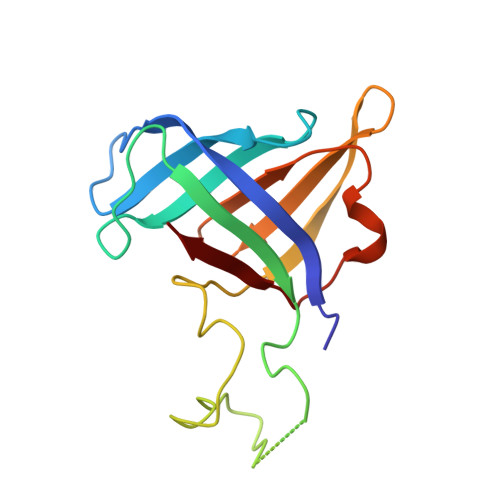
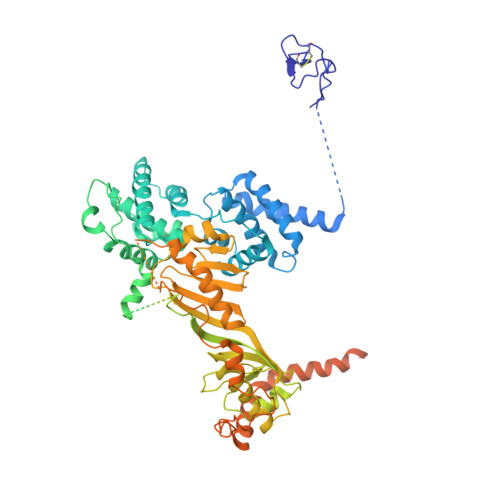
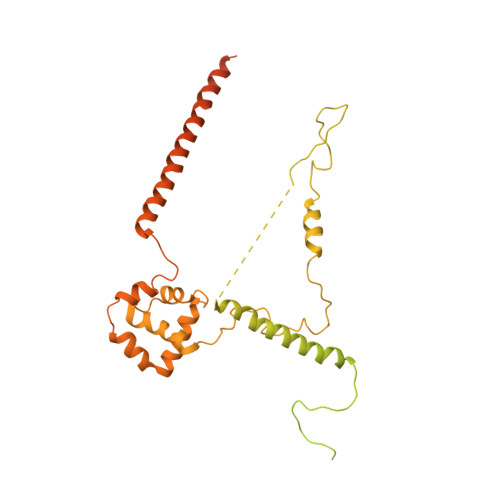
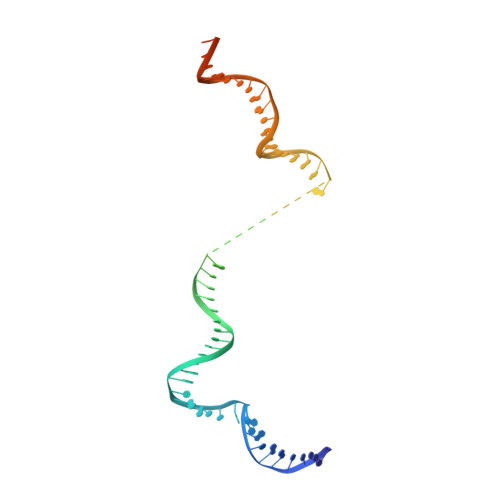
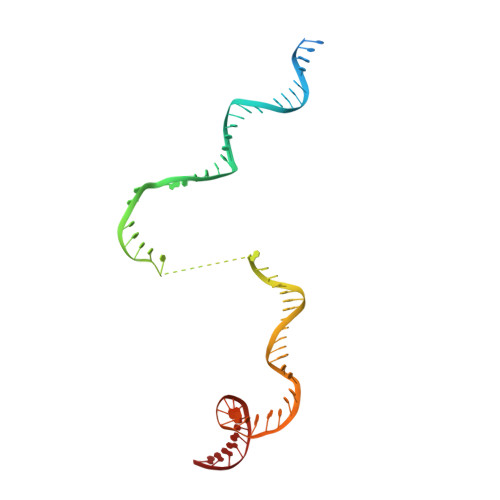
Structural visualization of RNA polymerase III transcription machineries.
Han, Y., Yan, C., Fishbain, S., Ivanov, I., He, Y.(2018) Cell Discov 4: 40-40
- PubMed: 30083386
- DOI: https://doi.org/10.1038/s41421-018-0044-z
- Primary Citation of Related Structures:
6CNB, 6CNC, 6CND, 6CNF - PubMed Abstract:
RNA polymerase III (Pol III) transcription initiation requires the action of the transcription factor IIIB (TFIIIB) and is highly regulated. Here, we determine the structures of Pol III pre-initiation complexes (PICs) using single particle cryo-electron microscopy (cryo-EM). We observe stable Pol III-TFIIIB complexes using nucleic acid scaffolds mimicking various functional states, in which TFIIIB tightly encircles the upstream promoter DNA. There is an intricate interaction between TFIIIB and Pol III, which stabilizes the winged-helix domains of the C34 subunit of Pol III over the active site cleft. The architecture of Pol III PIC more resembles that of the Pol II PIC than the Pol I PIC. In addition, we also obtain a 3D reconstruction of Pol III in complex with TFIIIB using the elongation complex (EC) scaffold, shedding light on the mechanism of facilitated recycling of Pol III prior to transcription re-initiation.
- 1Department of Molecular Biosciences, Northwestern University, Evanston, IL 60208 USA.
Organizational Affiliation: