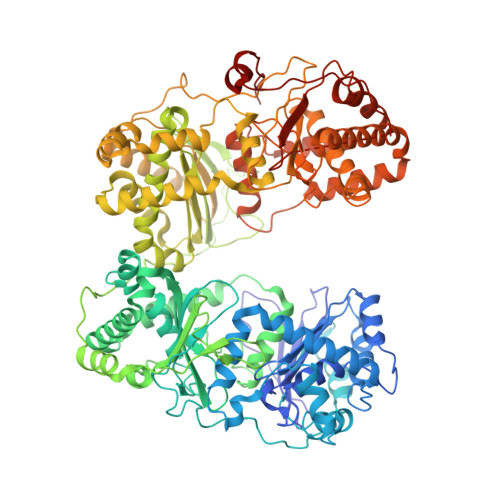
Ensemble cryoEM elucidates the mechanism of insulin capture and degradation by human insulin degrading enzyme.
Zhang, Z., Liang, W.G., Bailey, L.J., Tan, Y.Z., Wei, H., Wang, A., Farcasanu, M., Woods, V.A., McCord, L.A., Lee, D., Shang, W., Deprez-Poulain, R., Deprez, B., Liu, D.R., Koide, A., Koide, S., Kossiakoff, A.A., Li, S., Carragher, B., Potter, C.S., Tang, W.J.(2018) Elife 7
- PubMed: 29596046
- DOI: https://doi.org/10.7554/eLife.33572
- Primary Citation of Related Structures:
5WOB, 6B3Q, 6B70, 6B7Y, 6B7Z, 6BF6, 6BF7, 6BF8, 6BF9, 6BFC - PubMed Abstract:
Insulin degrading enzyme (IDE) plays key roles in degrading peptides vital in type two diabetes, Alzheimer's, inflammation, and other human diseases. However, the process through which IDE recognizes peptides that tend to form amyloid fibrils remained unsolved. We used cryoEM to understand both the apo- and insulin-bound dimeric IDE states, revealing that IDE displays a large opening between the homologous ~55 kDa N- and C-terminal halves to allow selective substrate capture based on size and charge complementarity. We also used cryoEM, X-ray crystallography, SAXS, and HDX-MS to elucidate the molecular basis of how amyloidogenic peptides stabilize the disordered IDE catalytic cleft, thereby inducing selective degradation by substrate-assisted catalysis. Furthermore, our insulin-bound IDE structures explain how IDE processively degrades insulin by stochastically cutting either chain without breaking disulfide bonds. Together, our studies provide a mechanism for how IDE selectively degrades amyloidogenic peptides and offers structural insights for developing IDE-based therapies.
- National Resource for Automated Molecular Microscopy, Simons Electron Microscopy Center, New York Structural Biology Center, New York, United States.
Organizational Affiliation: