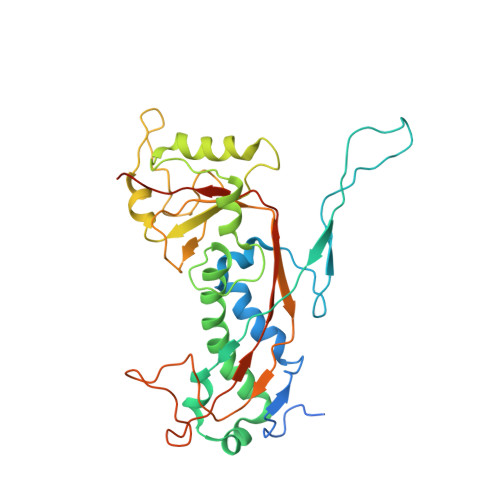
Competing scaffolding proteins determine capsid size during mobilization ofStaphylococcus aureuspathogenicity islands.
Dearborn, A.D., Wall, E.A., Kizziah, J.L., Klenow, L., Parker, L.K., Manning, K.A., Spilman, M.S., Spear, J.M., Christie, G.E., Dokland, T.(2017) Elife 6
- PubMed: 28984245
- DOI: https://doi.org/10.7554/eLife.30822
- Primary Citation of Related Structures:
6B0X, 6B23 - PubMed Abstract:
Staphylococcus aureus pathogenicity islands (SaPIs), such as SaPI1, exploit specific helper bacteriophages, like 80α, for their high frequency mobilization, a process termed 'molecular piracy'. SaPI1 redirects the helper's assembly pathway to form small capsids that can only accommodate the smaller SaPI1 genome, but not a complete phage genome. SaPI1 encodes two proteins, CpmA and CpmB, that are responsible for this size redirection. We have determined the structures of the 80α and SaPI1 procapsids to near-atomic resolution by cryo-electron microscopy, and show that CpmB competes with the 80α scaffolding protein (SP) for a binding site on the capsid protein (CP), and works by altering the angle between capsomers. We probed these interactions genetically and identified second-site suppressors of lethal mutations in SP. Our structures show, for the first time, the detailed interactions between SP and CP in a bacteriophage, providing unique insights into macromolecular assembly processes.
- Protein Expression Laboratory, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, United States.
Organizational Affiliation: