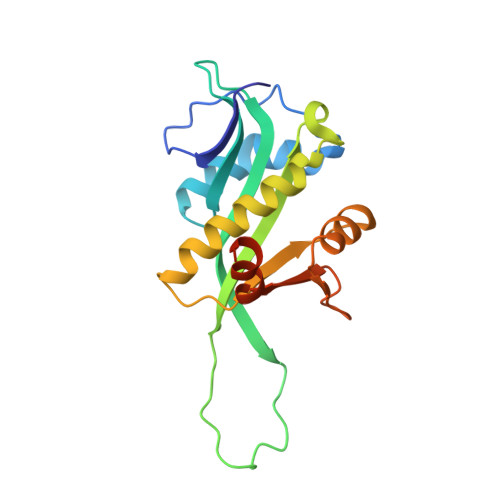
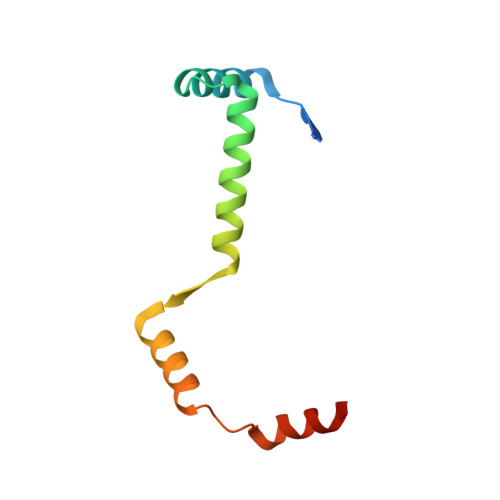
Crystal Structure of the Enterohemorrhagic Escherichia coli AtaT-AtaR Toxin-Antitoxin Complex.
Yashiro, Y., Yamashita, S., Tomita, K.(2019) Structure 27: 476
- PubMed: 30612860
- DOI: https://doi.org/10.1016/j.str.2018.11.005
- Primary Citation of Related Structures:
6AJM, 6AJN - PubMed Abstract:
AtaT-AtaR is an enterohemorrhagic Escherichia coli toxin-antitoxin system that modulates cellular growth under stress conditions. AtaT and AtaR act as a toxin and its repressor, respectively. AtaT is a member of the GNAT family, and the dimeric AtaT acetylates the α-amino group of the aminoacyl moiety of methionyl initiator tRNA fMet , thereby inhibiting translation initiation. The crystallographic analysis of the AtaT-AtaR complex revealed that the AtaT-AtaR proteins form a heterohexameric [AtaT-(AtaR 4 )-AtaT] complex, where two V-shaped AtaR dimers bridge two AtaT molecules. The N-terminal region of AtaR is required for its dimerization, and the C-terminal region of AtaR interacts with AtaT. The two AtaT molecules are spatially separated in the AtaT-AtaR complex. AtaT alone forms a dimer in solution, which is enzymatically active. The present structure, in which AtaR prevents AtaT from forming an active dimer, reveals the molecular basis of the AtaT toxicity repression by the antitoxin AtaR.
- Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, Kashiwa, Chiba 277-8562, Japan.
Organizational Affiliation: