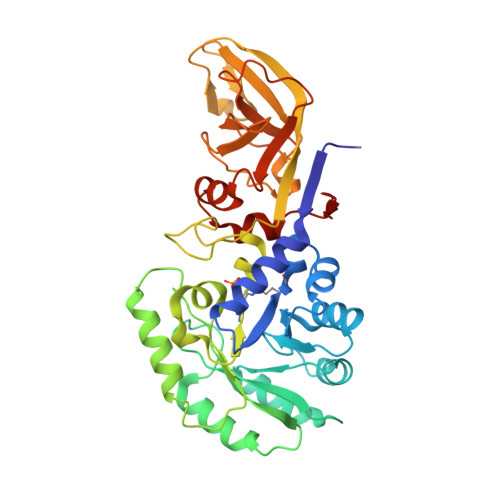
Enzymatic characterization and crystal structure of biosynthetic alanine racemase from Pseudomonas aeruginosa PAO1.
Dong, H., Han, Q., Guo, Y., Ju, J., Wang, S., Yuan, C., Long, W., He, X., Xu, S., Li, S.(2018) Biochem Biophys Res Commun 503: 2319-2325
- PubMed: 29964014
- DOI: https://doi.org/10.1016/j.bbrc.2018.06.155
- Primary Citation of Related Structures:
6A2F - PubMed Abstract:
Alanine racemase is a pyridoxal-5'-phosphate (PLP)-dependent enzyme that reversibly catalyzes the conversion of l-alanine to d-alanine. d-alanine is an essential constituent in many prokaryotic cell structures. Inhibition of alanine racemase is lethal to prokaryotes, creating an attractive target for designing antibacterial drugs. Here we report the crystal structure of biosynthetic alanine racemase (Alr) from a pathogenic bacteria Pseudomonas aeruginosa PAO1. Structural studies showed that P. aeruginosa Alr (PaAlr) adopts a conserved homodimer structure. A guest substrate d-lysine was observed in the active site and refined to dual-conformation. Two buffer ions, malonate and acetate, were bound in the proximity to d-lysine. Biochemical characterization revealed the optimal reaction conditions for PaAlr.
- Key Laboratory of Tianjin Radiation and Molecular Nuclear Medicine, Institute of Radiation Medicine, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, 300192, China.
Organizational Affiliation: