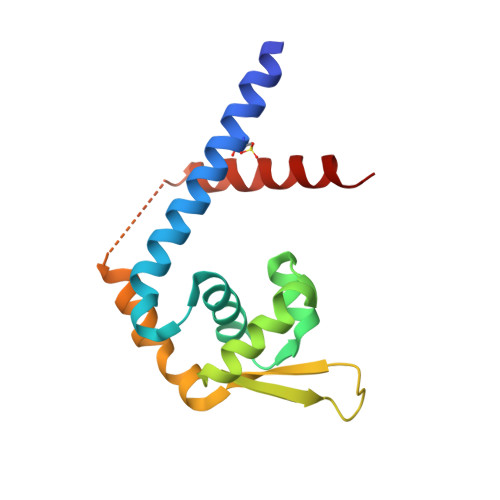
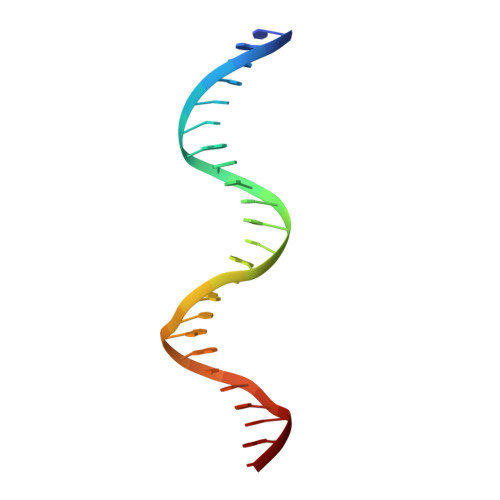
Structural Insights into the Redox-Sensing Mechanism of MarR-Type Regulator AbfR.
Liu, G., Liu, X., Xu, H., Liu, X., Zhou, H., Huang, Z., Gan, J., Chen, H., Lan, L., Yang, C.G.(2017) J Am Chem Soc 139: 1598-1608
- PubMed: 28086264
- DOI: https://doi.org/10.1021/jacs.6b11438
- Primary Citation of Related Structures:
5HLG, 5HLH, 5HLI - PubMed Abstract:
As a master redox-sensing MarR-family transcriptional regulator, AbfR participates in oxidative stress responses and virulence regulations in Staphylococcus epidermidis. Here, we present structural insights into the DNA-binding mechanism of AbfR in different oxidation states by determining the X-ray crystal structures of a reduced-AbfR/DNA complex, an overoxidized (Cys13-SO 2 H and Cys13-SO 3 H) AbfR/DNA, and 2-disulfide cross-linked AbfR dimer. Together with biochemical analyses, our results suggest that the redox regulation of AbfR-sensing displays two novel features: (i) the reversible disulfide modification, but not the irreversible overoxidation, significantly abolishes the DNA-binding ability of the AbfR repressor; (ii) either 1-disulfide cross-linked or 2-disulfide cross-linked AbfR dimer is biologically significant. The overoxidized species of AbfR, resembling the reduced AbfR in conformation and retaining the DNA-binding ability, does not exist in biologically significant concentrations, however. The 1-disulfide cross-linked modification endows AbfR with significantly weakened capability for DNA-binding. The 2-disulfide cross-linked AbfR adopts a very "open" conformation that is incompatible with DNA-binding. Overall, the concise oxidation chemistry of the redox-active cysteine allows AbfR to sense and respond to oxidative stress correctly and efficiently.
- Laboratory of Chemical Biology, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences , Shanghai 201203, China.
Organizational Affiliation: