Crystal structure of EGFR 696-1022 T790M/C797S in complex with D3003
Zhu, S.J., Yun, C.H.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Epidermal growth factor receptor | 331 | Homo sapiens | Mutation(s): 2 Gene Names: EGFR, ERBB, ERBB1, HER1 EC: 2.7.10.1 | 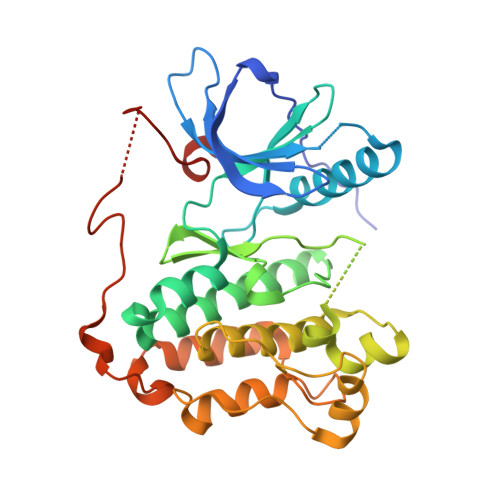 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P00533 (Homo sapiens) Explore P00533 Go to UniProtKB: P00533 | |||||
PHAROS: P00533 GTEx: ENSG00000146648 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00533 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 9JO Query on 9JO | B [auth A] | N-{trans-4-[3-(2-chlorophenyl)-7-{[3-methyl-4-(4-methylpiperazin-1-yl)phenyl]amino}-2-oxo-3,4-dihydropyrimido[4,5-d]pyrimidin-1(2H)-yl]cyclohexyl}propanamide C33 H41 Cl N8 O2 WVLWGBZNXIVAKC-YOCNBXQISA-N |  | ||
| CL Query on CL | C [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 148.731 | α = 90 |
| b = 148.731 | β = 90 |
| c = 148.731 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |