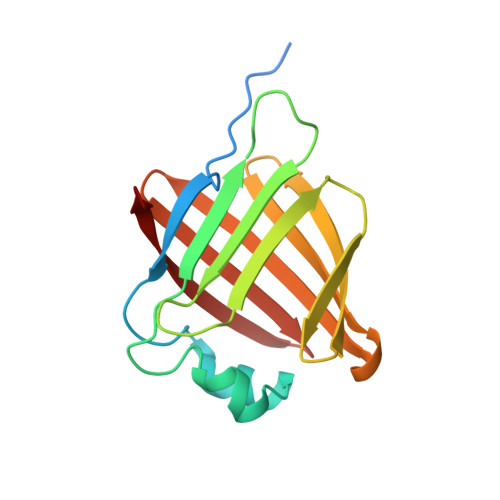
Crystal structure of secretory abundant heat soluble protein 4 from one of the toughest "water bears" micro-animals Ramazzottius Varieornatus
Fukuda, Y., Inoue, T.(2018) Protein Sci 27: 993-999
- PubMed: 29493034
- DOI: https://doi.org/10.1002/pro.3393
- Primary Citation of Related Structures:
5Z4G - PubMed Abstract:
Though anhydrobiotic tardigrades (micro-animals also known as water bears) possess many genes of secretory abundant heat soluble (SAHS) proteins unique to Tardigrada, their functions are unknown. A previous crystallographic study revealed that a SAHS protein (RvSAHS1) from one of the toughest tardigrades, Ramazzottius varieornatus, has a β-barrel architecture similar to fatty acid binding proteins (FABPs) and two putative ligand binding sites (LBS1 and LBS2) where fatty acids can bind. However, some SAHS proteins such as RvSAHS4 have different sets of amino acid residues at LBS1 and LBS2, implying that they prefer other ligands and have different functions. Here RvSAHS4 was crystallized and analyzed under a condition similar to that for RvSAHS1. There was no electron density corresponding to a fatty acid at LBS1 of RvSAHS4, where a putative fatty acid was observed in RvSAHS1. Instead, LBS2 of RvSAHS4, which was composed of uncharged residues, captured a putative polyethylene glycol molecule. These results suggest that RvSAHS4 mainly uses LBS2 for the binding of uncharged molecules.
- Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871, Japan.
Organizational Affiliation: