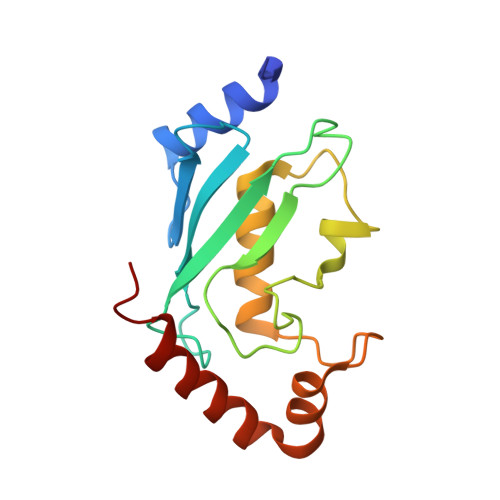
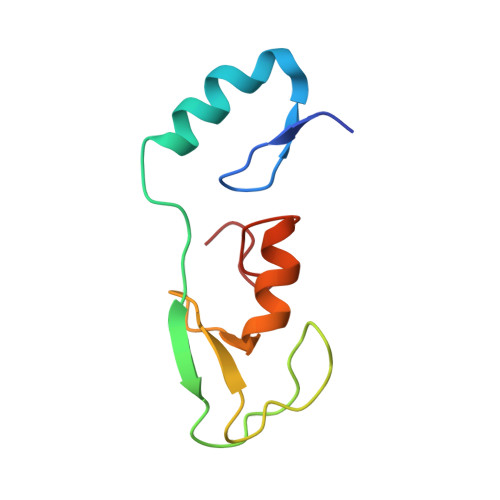
Structural insights into the nanomolar affinity of RING E3 ligase ZNRF1 for Ube2N and its functional implications.
Behera, A.P., Naskar, P., Agarwal, S., Banka, P.A., Poddar, A., Datta, A.B.(2018) Biochem J 475: 1569-1582
- PubMed: 29626159
- DOI: https://doi.org/10.1042/BCJ20170909
- Primary Citation of Related Structures:
5YWR - PubMed Abstract:
RING ( R eally I nteresting N ew G ene) domains in ubiquitin RING E3 ligases exclusively engage ubiquitin (Ub)-loaded E2s to facilitate ubiquitination of their substrates. Despite such specificity, all RINGs characterized till date bind unloaded E2s with dissociation constants ( K d s) in the micromolar to the sub-millimolar range. Here, we show that the RING domain of E3 ligase ZNRF1, an essential E3 ligase implicated in diverse cellular pathways, binds Ube2N with a K d of ∼50 nM. This high-affinity interaction is exclusive for Ube2N as ZNRF1 interacts with Ube2D2 with a K d of ∼1 µM, alike few other E3s. The crystal structure of ZNRF1 C-terminal domain in complex with Ube2N coupled with mutational analyses reveals the molecular basis of this unusual affinity. We further demonstrate that the ubiquitination efficiency of ZNRF1 : E2 pairs correlates with their affinity. Intriguingly, as a consequence of its high E2 affinity, an excess of ZNRF1 inhibits Ube2N-mediated ubiquitination at concentrations ≥500 nM instead of showing enhanced ubiquitination. This suggests a novel mode of activity regulation of E3 ligases and emphasizes the importance of E3-E2 balance for the optimum activity. Based on our results, we propose that overexpression-based functional analyses on E3 ligases such as ZNRF1 must be approached with caution as enhanced cellular levels might result in aberrant modification activity.
- Department of Biochemistry, Bose Institute, P-1/12 CIT Scheme VIIM, Kolkata 700054, India.
Organizational Affiliation: