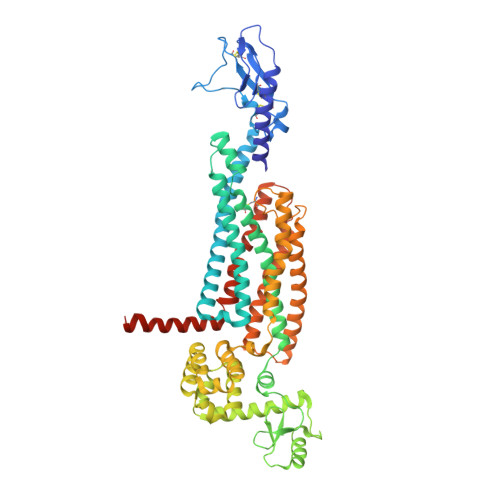
Structure of the glucagon receptor in complex with a glucagon analogue.
Zhang, H., Qiao, A., Yang, L., Van Eps, N., Frederiksen, K.S., Yang, D., Dai, A., Cai, X., Zhang, H., Yi, C., Cao, C., He, L., Yang, H., Lau, J., Ernst, O.P., Hanson, M.A., Stevens, R.C., Wang, M.W., Reedtz-Runge, S., Jiang, H., Zhao, Q., Wu, B.(2018) Nature 553: 106-110
- PubMed: 29300013
- DOI: https://doi.org/10.1038/nature25153
- Primary Citation of Related Structures:
5YQZ - PubMed Abstract:
Class B G-protein-coupled receptors (GPCRs), which consist of an extracellular domain (ECD) and a transmembrane domain (TMD), respond to secretin peptides to play a key part in hormonal homeostasis, and are important therapeutic targets for a variety of diseases. Previous work has suggested that peptide ligands bind to class B GPCRs according to a two-domain binding model, in which the C-terminal region of the peptide targets the ECD and the N-terminal region of the peptide binds to the TMD binding pocket. Recently, three structures of class B GPCRs in complex with peptide ligands have been solved. These structures provide essential insights into peptide ligand recognition by class B GPCRs. However, owing to resolution limitations, the specific molecular interactions for peptide binding to class B GPCRs remain ambiguous. Moreover, these previously solved structures have different ECD conformations relative to the TMD, which introduces questions regarding inter-domain conformational flexibility and the changes required for receptor activation. Here we report the 3.0 Å-resolution crystal structure of the full-length human glucagon receptor (GCGR) in complex with a glucagon analogue and partial agonist, NNC1702. This structure provides molecular details of the interactions between GCGR and the peptide ligand. It reveals a marked change in the relative orientation between the ECD and TMD of GCGR compared to the previously solved structure of the inactive GCGR-NNC0640-mAb1 complex. Notably, the stalk region and the first extracellular loop undergo major conformational changes in secondary structure during peptide binding, forming key interactions with the peptide. We further propose a dual-binding-site trigger model for GCGR activation-which requires conformational changes of the stalk, first extracellular loop and TMD-that extends our understanding of the previously established two-domain peptide-binding model of class B GPCRs.
- CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Pudong, Shanghai 201203, China.
Organizational Affiliation: