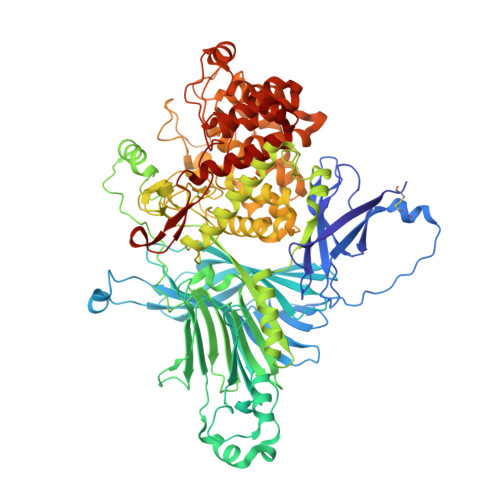
Crystal structure of exo-rhamnogalacturonan lyase from Penicillium chrysogenum as a member of polysaccharide lyase family 26
Kunishige, Y., Iwai, M., Nakazawa, M., Ueda, M., Tada, T., Nishimura, S., Sakamoto, T.(2018) FEBS Lett 592: 1378-1388
- PubMed: 29574769
- DOI: https://doi.org/10.1002/1873-3468.13034
- Primary Citation of Related Structures:
5XQ3, 5XQG, 5XQJ, 5XQO - PubMed Abstract:
Exo-rhamnogalacturonan lyase from Penicillium chrysogenum 31B (PcRGLX) was recently classified as a member of polysaccharide lyase (PL) family 26 along with hypothetical proteins derived from various organisms. In this study, we determined the crystal structure of PcRGLX as the first structure of a member of this family. Based on the substrate-binding orientation and substrate specificity, PcRGLX is an exo-type PL that cleaves rhamnogalacturonan from the reducing end. Analysis of PcRGLX-complex structures with reaction products indicate that the active site possesses an L-shaped cleft that can accommodate galactosyl side chains, suggesting side-chain-bypassing activity in PcRGLX. Furthermore, we determined the residues critical for catalysis by analyzing the enzyme activities of inactive variants.
- Division of Applied Life Sciences, Graduate School of Life and Environmental Sciences, Osaka Prefecture University, Sakai, Japan.
Organizational Affiliation: