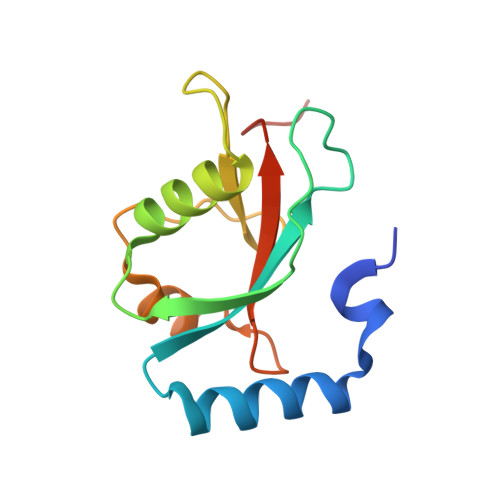
The crystal structure of mouse LC3B in complex with the FYCO1 LIR reveals the importance of the flanking region of the LIR motif
Sakurai, S., Tomita, T., Shimizu, T., Ohto, U.(2017) Acta Crystallogr F Struct Biol Commun 73: 130-137
- PubMed: 28291748
- DOI: https://doi.org/10.1107/S2053230X17001911
- Primary Citation of Related Structures:
5WRD - PubMed Abstract:
FYVE and coiled-coil domain-containing protein 1 (FYCO1), a multidomain autophagy adaptor protein, mediates microtubule plus-end-directed autophagosome transport by interacting with kinesin motor proteins and with the autophagosomal membrane components microtubule-associated protein 1 light chain 3 (LC3), Rab7 and phosphatidylinositol 3-phosphate (PI3P). To establish the structural basis for the recognition of FYCO1 by LC3, the crystal structure of mouse LC3B in complex with the FYCO1 LC3-interacting region (LIR) motif peptide was determined. Structural analysis showed that the flanking sequences N-terminal and C-terminal to the LIR core sequence of FYCO1, as well as the tetrapeptide core sequence, were specifically recognized by LC3B and contributed to the binding. Moreover, comparisons of related structures revealed a conserved mechanism of FYCO1 recognition by different LC3 isoforms among different species.
- Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.
Organizational Affiliation: